Before jumping into the technical details, view EPCM’s list of Cathodic Protection Projects.
The fundamental operational principles of Cathodic Protection were made possible by explaining the phenomenon of corrosion caused by the passage of metal ions into the solution of its surrounding environments. When a metal or alloy is immersed in an electrolyte (this can be soil, a liquid, or a mixture of these two) certain areas of the metal start to function as an anode whilst the rest of the surface will act as the cathode of this electrical cell. At the anodic sites metal atoms in the crystalline lattice are transferred into metal ions in this corrosive environment. The oxidation reaction is commonly called the anodic reaction and the reduction reaction is called the cathodic reaction. Both electrochemical reactions are required for corrosion to occur.
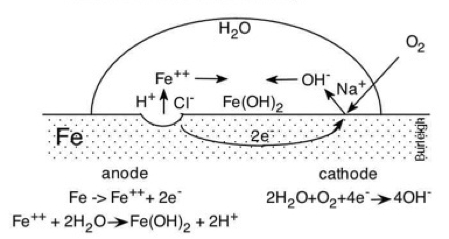
Figure 1: Corrosion of metal in a waterdrop
The site where the metal is being oxidized is referred to as the anode or anodic site. At this site, direct electric current (defined as a positive flow of charge) flows from the metal surface into the electrolyte as the metal ions leave the surface. This current flow in the electrolyte to the site where oxygen, water, or some other species is being reduced. This site is referred to as the cathode or cathodic site.
There are four necessary parts for a corrosion cell.
- There must be an anode
- There must be a cathode
- There must be an electrical path connecting the anode and the cathode.
- The anode and cathode must be immersed in an electrically conductive electrolyte. This will almost always be moist soil or a body of water.
1 How To Detect Corrosion
The electrochemical nature of the corrosion process gives us the ability to detect and mitigate corrosion of structures. When a metal is placed in an electrolyte there will be a potential difference. We can measure and monitor this voltage using a Reference Electrode to determine the corrosion potential or native potential for that structure in the specific environment.
2 How To Mitigate Corrosion
Coating of the structures, be it a pipeline, tank bottom, steel support column or buried tank can be very effective against corrosion. The primary function of coating systems is to isolate the metallic (e.g. steel) surface from the corrosive electrolyte (e.g. seawater, soil, etc.,) and to minimise the amount of current required for Cathodic Protection. The coating is termed the primary corrosion protection system on most submerged structures. The only problem with coatings is that they can never be 100% perfect and there will always be manufacturers or application errors. Coatings are also subject to degradation, soil stresses and movement, damage from third parties and disbondment. Accelerated corrosion on small “defects” in the coating can lead to leaks or ruptures in the pipeline even where the coating protects a large area of the structure. For this reason, Cathodic Protection is always advised to protect these exposed sections as well as any other areas that may become evident during operation of the system and hence Cathodic Protection is referred to as supplementary protection.
3 How Does Cathodic Protection Work
Cathodic Protection works by forcing Direct Current into the structure that requires protection. This causes the current in the negative direction. When done correctly the current flowing will overpower the corrosion current that would naturally be discharged and cause corrosion. The entire surface will become a cathode and the corrosion rate will be reduced. The level of Cathodic Protection required must be calculated based on various design, coating and environmental factors.
4 What Systems Can Be Used
To allow current to flow through the structure that requires protection an electrical connection is required. The simplest way of doing this is to attach a conductor. If the structure is visible above ground this can be done quite easily, however, this is a bit more challenging with buried structures like tanks and pipelines. To attach cables a few techniques are available.
4.1 Galvanic Anodes.
Galvanic Anodes are materials with a native potential lower than that of the material requiring protection. When these materials are electrically connected the one will form an anode and the other a cathode as seen in Fig. 2. The metal corroding will be the Sacrificial Anode purposefully selected to protect the structure that requires Cathodic Protection. Based on environmental conditions there are a few options of materials to use like Aluminium, Zinc and Magnesium either pure or as an Alloy. The most practical way of connecting the sacrificial anode to the pipe would be to terminate the connections in a box or enclosure above ground. This is usually referred to as a Test Post or more descriptive to the application a West Point. This facility allows the technician to take pipe potential measurements periodically. If a shunt resistor is used in the termination, a current measurement can be taken with the same equipment used for the pipe potential measurement during maintenance and auditing. To increase the total cross-sectional area of the anodes and improve the connection to ground, these types on anodes when buried are typically encased in a conductive backfill medium. This will be made up of Bentonite, gypsum and sodium sulphate (typically 70/25/5 ratio) and all will be placed in a cotton bag. Bentonite is hygroscopic so it will draw and retain moisture from the environment. This will help to lower the resistance to ground for better performance of the system.

Figure 2: Galvanic anode cathodic protection
4.2 Impressed Current Cathodic Protection (ICCP)
In some instances, the small driving voltage that a Galvanic Anode can provide will not be adequate to protect certain structures. In this instance, an external power source can be used to remedy this as seen in Fig 3.

Figure 3: Impressed current cathodic protection
These Direct Current power sources are called Rectifiers. They are available in different power outputs, most commonly 10 to 50V DC with a Current output suitable for the system it is intended to protect. These Rectifiers are manufactured to maintain a predetermined level of protection. The main feedback control used in the industry is either Constant Current (CC), Constant Potential (CP) or Constant Voltage (CV). Each of these has its application based on environmental factors. Stray currents can play havoc on the operation of a CP system, so the reliability and controllability of the rectifier play a key role in assuring protection and selecting the correct control. The rectifier will be connected between the structure and ground bed. Power will be forced through a ground bed made up of multiple anodes buried in the ground. This will effectively cause the anodes to corrode and protect the structure. These anodes are buried in a conductive backfill to decrease the overall resistance of the system. Several types of backfill can be used, mostly carbonaceous. The backfill acts to increase the cross-sectional area of the anode, but also extends the anode life as it decreases the anodes consumption rate. There are several types of anodes available. Most popular are high silicon cast iron, Mixed Metal Oxide (MMO), platinum and graphite.
5 How Cathodic Protection Affects Coating
Because the coating covers the biggest parts of the structure, the current will only be required to flow to the exposed areas. The coating will also allow current to flow through it, this based on the type of coating, the material used and the applied thickness. Since no coating can be a perfect insulator the resistivity must be taken into consideration when designing a system. Another factor to take into consideration is that excessive amounts of Cathodic Protection current may cause damage to the coating.
This process is called Cathodic Disbondment. Cathodic Disbondment can be eliminated by correct design of the system and by maintaining the Cathodic Protection system to operate within design parameters.
6 How Cathodic Protection Affects Other structures
Neighbouring buried steel structures can be susceptible to stray current corrosion caused by a Cathodic Protection system. It is important to consider all foreign services when designing a CP system to avoid this type of interference. This form of stray current damage is most commonly caused by impressed current CP systems as larger amounts of current can be forced to travel through the ground bed
7 How To Decide What System To Use
Some of the main factors that determine the type of installation required are as follows.
- The corrosivity of the environment. This is determined by soil testing.
- The resistivity and type of soil in which the structure will be installed.
- Is the structure coated or bare? The type of coating, thickness, quality of the application and how will it be affected by the environment etc.
- The material the structure is made of.
- The total size of the system that requires protection.
- Any other Cathodic Protection systems close by that can cause interference. Any foreign metal structures that could shield against CP.
- Could there be interference from stray currents?
8 Electrical Shielding and Stray Currents
An electrical shield is any barrier that prevents the flow of current intended to protect the structure from flowing in the electrolyte (water and soil) surrounding the structure. There are two main types of shielding. One is a non-metallic barrier that serves as an electrical insulator. The other barrier diverts the current by passing it onto other metallic structures that do not form part of the protected structure.
8.1 Nonmetallic Shielding
Some of the most common causes of nonmetallic shielding are where the coating has been disbonded and water ingress has occurred between the steel surface and the coating.
This section will not be protected as there is no flow of electrical current to protect the steel surface. Another scenario is where a pipe has been installed in a nonconductive casing under a road or river. If the casing is inadvertently flooded with water corrosion can occur.
8.2 Metallic Shielding
Some examples of metallic shielding. When the wire reinforcing in concrete is connected to the steel structure accidentally. Even though this is not a solid barrier most of the current will flow to the reinforcing steel. Even one connected wire is enough to severely cripple the efficacy of the CP system in that area where there is reinforcing wire. Usually, the interconnected reinforcing wire is interconnected into a mesh or cage. If a steel casing was used (for example a road crossing or river) but the steel structure is in electrical contact with the casing, the current will flow from this connection point to the outside of the casing. If the casing is left uncoated and there is no electrical continuity to the protected structure, but it has been filled with water unintentionally current will continue to flow through the casing and cause the casing to corrode and not the protected structure. Shielding can also be caused in heavily congested areas. Tank farms, pump stations and plant or production areas can have several buried metal structures. Special design requirements and measurement techniques must be applied to these sections to assure the Cathodic Protection system performs as required.
8.3 Stray Current
Stray currents can be caused by other Cathodic Protection systems in the vicinity as well as DC railroad systems. Interference from foreign services is not too difficult to manage as the current flow will in most cases be consistent. Installing cross bonding conductors with resistive link panels will in most cases solve these problems. The problem is caused as the electrical current will always choose the easiest path to follow. When a pipeline runs between foreign service and the ground bed designed to protect that foreign service structure, it can cause current to be forced onto your system. Having, for instance, a steel pipeline running in running in the direction of this current will cause current to flow onto the pipeline through any coating defects and when the path of least resistance ends it will flow back into the surrounding soil. This point can make the steel surface extremely anodic and cause massive corrosion in short amounts of time. Railroad systems using DC can be an even bigger problem. The large driving voltages and currents also tend to be erratic. The amount of current flow depends on the traffic on the rail as well as the size of the train in operation at that time. This can cause your system to either go anodic as the Rectifier installed can not provide enough current to overcome this interference or it can go very cathodic and this may cause disbondment among other issues.
In most of these instances, an FDU (Forced Drainage Unit) or NDU (Natural Drainage Unit) will be installed to help solve this problem. An FDU is a rectifier with a parallel diode connected to the output. If the structure becomes anodic the diode will allow current to be drained to the ground bed and not allow it to flow naturally into the ground causing corrosion. An NDU is just a diode with a drain point to do the same thing. On both these types of installations, the FDU or NDU can also be connected between the structure and railroad causing the interference.
9 Measurements and maintenance
There most frequent test performed in the corrosion industry is a potential measurement with a Copper Sulphate Reference Electrode (referenced to as CSE). The electrode is placed in damp soil close to the structure for close measurements and or at a distance or remotely for remote measurements (Fig. 4). A potential measurement can give in indication to the extent of the corrosion, identify hot spots for corrosion and stray current electrolysis.

Figure 4: Measurement of a pipe potential using a reference electrode
9.1 Coupon Measurements
Sometimes a coupon is buried close to the structure to help determine the level of protection. A coupon is a small piece of bare steel connected electrically to the structure. It is made up of similar grade as that of the structure and is buried near the structure to mimic the soil conditions on any coating defects. The use of a coupon allows the technician to determine the amount of protection being offered to the structure and if terminated in an accessible enclosure it can be temporarily disconnected to evaluate the polarization level of the structure. The current flowing through the coupon can give you the current density of the exposed steel if the size of the coupon is known.
This information is invaluable, and it is recommended to keep a record of testing done on a maintenance schedule.
9.2 Reference Electrode
The Reference Electrode (RE) is one of the most valuable tools in the toolbox of a CP technician. It allows a structure to electrolyte measurement to be taken without interference by heat or changing soil conditions.
It is what helps him to determine if a CP system is working and can be useful in fault finding too. There are a few different types of reference electrodes.
The Copper Sulphate Electrode (CSE) is one of the oldest RE and is mainly used in the CP industry Fig. 5. It is a copper rod, submerged in a saturated solution of Copper Sulphate. It has a porous plug to facilitate conduction to the electrolyte it is placed in.

Figure 5: Copper sulphate reference electrode
Care should be taken to avoid contamination and to keep the RE clean. It can be contaminated with brackish water through osmosis when exposed for extended periods. Do not leave it in the sun and try to avoid extreme temperatures.
The Silver Chloride Electrode is a laboratory-grade electrode used for many electrochemical environments making it suitable to use in seawater. It is made with a silver wire coated in silver chloride, submerged in a solution of potassium chloride with a porous plug to connect to the electrolyte. This type of electrode is simple to construct, inexpensive to manufacture and has a very stable potential using non-toxic components.
The Saturated Calomel Electrode (SCE) is based on the reaction between mercury and mercury chloride. It is more resistant to contamination than a Silver Chloride Electrode. This type of electrode is rarely used for on-site measurement as there is a risk of mercury contamination if it breaks.
10 Cable Connections
One of the critical items in any Cathodic Protection system is cable connections to the steel structure. These make provision for anode connections, connection to a rectifier or to facilitate measurements. A cable connection must be made with minimal electrical resistance and insulated or wrapped correctly as the coating will be removed in most cases to do a connection.
Pin Brazing is an older technique to attach a metal stud for a cable connection. It uses a Silver Solder alloy pin that is soldered to the structure using a high current electric arc as a heating source. This method heats the pin and the area where the pin is to be attached. It starts by drawing an arc and after about 2 seconds pushes the molten metal together to form a connection. This method generates quite a lot of heat on the weld surface, so it is not the first choice in connecting to steel pipelines.
Exothermic welding is another process well known in the industry. It uses a graphite or carbon mould and powder of copper oxide and aluminium. The mould is designed to have a crucible and a mould. The crucible part is filled with exothermic powder. The powder is kept in the crucible with a metal disk that is designed to melt once at welding temperature, then release the molten metal into the mould underneath. The mould cavity comes in many configurations to suit a variety of application. This technique can even join dissimilar metals. It is used often on the manufacturing of earth mats. This method also generates lots of heat.
Capacitive Discharge (CD) stud welding is most commonly used in the connection of cables to steel pipelines. It is the preferred method as it generates very little heat, does not have a big area affected by heat, it is fast and reliable. This technique is also approved by various pipeline operators to be used whilst the pipe is in operation. It works by discharging energy stored in capacitors to draw an arc, heat the bottom of the stud and the area of the structure. These two are then forced together for the metal to re-solidify. The welding process takes a few milliseconds, so there is very little heat to transfer into the weld area.

 To all knowledge
To all knowledge