1 Covid-19 The Burning Platform for Ventilators Now
1.1 A Global Pandemic
At first, the world thought it was “just” the flue and far away in China. When it hit Italy, the realisation occurred that is was different than the seasonal flu. Then the director-general of the World Health Organisation (WHO), Dr Tedros Adhanom Ghebreyesus declared the COVID-19 virus a global pandemic on the 11th of March 2020.
COVID-19 differs from the seasonal flue as it is highly infectious. Data from the WHO illustrates the exponential growth of the infection rate:
67 days to 100.000 cases
11 days to 200.000 case
4 days to 300.000 cases
The second major difference is that COVID-19 is a more severe disease than seasonal influenza. Our immune systems have been trained against the seasonal flu, yet there is no immunity against the novel COVID-19 virus. With the result that more people are susceptible to infection, and some will suffer severe disease.
Globally, about 3.4% of reported COVID-19 cases have died. By comparison, seasonal flu generally kills far fewer than 1% of those infected. According to Dr Anthony Fauci, director of the National Institutes of Allergy and Infectious Disease in the USA, COVID-19 is 10 times more lethal than the seasonal flu.
Thirdly, we have vaccines and therapeutics for seasonal flu, but no available vaccines or medicine to either prevent or combat COVID-19. However, scientists worldwide are working around the clock to clinically trial vaccines.
1.2 Not Enough Ventilators
The experience in China, Italy and Spain and the modelling used by mathematicians around the world, indicate the number of people who will become critically ill with COVID-19 will greatly exceed the capacity to care for them using respiratory support.
A retrospective cohort study on the clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China give hard facts. This study concluded that 17% of patients infected with COVID-19 require invasive mechanical ventilation. (Fei Zhou, 2020)
Using the quantitative data available and extrapolating it across the world population the world must come to terms with the fact that there are too few high-performance ventilators in the world to simultaneously treat the many severe COVID-19 cases. All over the world, bottlenecks could arise if the measures taken now do not work and a high number of serious cases of illness occur.
COVID-19 will not discriminate, both rich and poor countries will be hit hard.
1.3 NEITHER FOR THE RICH
In one of the world’s richest countries, the UK has 5,000 ventilators in operation and a couple of hundred in stock, but in a worst-case scenario, they could need about 20,000 of these machines to save lives.
New York with a population of 19 million people have a couple of thousands of ventilators in operation but predictions from several sources indicate that they will need approximately 30 000 – 40 0000 when the virus reaches its apex end of April 2020. Failure to provide these ventilators will have the consequence that patients will need to be manually ventilated with bag valve masks – if there are enough. Watch a clip of the governor of New York, Andrew Cuomo proclaiming that the fight against the Coronavirus all comes down to the provision of a sufficient number of ventilators.
1.4 Nor For The Poor
The situation is especially critical in poorer countries with weak healthcare systems and weaker buying power. In the West African country of Mali, home to also almost 19 million people, there are 56 ventilators available.
In South Africa, a country with a population of almost 54 million people there are an estimated 4 000 ventilators in the private health sector, and about half that number in the public sector according to an online article by The Citizen newspaper. News24 reported that Marius Fourie, the managing director of Draeger South Africa said that Africa was unfortunately at the back of the line for ventilators. Draeger South Africa is the subsidiary of one of the world’s largest ventilator manufacturers in Germany.
India, by most estimates, only has 48,000 ventilators. Nobody quite knows how many of these breathing assistance machines are in operation. It could be assumed that all those available are being used in intensive care units on existing patients with other diseases.
1.5 A Race Against The Clock
As the infection rate is skyrocketing and global demand is surging the provision of ventilators is clearly a race against the clock. The situation is further exacerbated by broken supply chains. The global supply chain of ventilator parts has been heavily impacted by the shutdown of Chinese factories as most conventional manufacturers heavily rely on the production of components in China. Further, airlifting of parts, components and manufactured ventilators is a challenge as most airlines have shut down operations. In the race against time, sea freight is not an option anymore.
Even though the major manufactures all pledged to massively ramp up production, the supply will not be able to satisfy the unbelievable demand created by COVID-19. The situation is particularly adverse for developing countries as they are most likely to be last in the ventilator queue as suppliers, headquartered in developed countries will most likely have an obligation to serve them first.
2 Ventilators for Developed Countries
2.1 HIGH-TECH VENTILATORMANUFACTURERS
There are several international companies manufacturing state of the art ventilator machines and equipment. These companies mostly source from a variety of specialized producers and rely on a very skilled and specialised workforce with an interconnected global supply chain.
These state of the art ventilators most found in hospital ICUs are sophisticated and according to Medtronic can cost between $25,000 and $50,000. Factors that can contribute to this price range include user configurability options and built-in safety features. If a hospital decides to buy a ventilator is also need to take into account additional costs for staff and training in order to operate the machine.
2.1.1 DRAEGER
Dräger is an international leader in the fields of medical and safety technology.
Top Draeger ventilators are the Evita Infinity V500, Savina 300 Select, Savina 300 and Oxylog 3000 designed for ventilation during transport.
Draeger’s reaction to the COVID-19 crisis is that they are as of the end of March 2020 producing almost twice as many ventilators as before and are in the process of expanding their production capacity even further. This is more than 50 per cent for one type of device, which is used extensively in connection with ventilation therapy for COVID-19 patients. Yet Draeger is warning that limitations on for example test rooms for ventilators might hinder an increase in production.
2.1.2 GE Health
Since the COVID-19 outbreak began, GE Healthcare has doubled its capacity of ventilator production and has plans to double it again by end of Q2 2020 to address unprecedented demand.
GE Health is the manufacturer of the CARESCAPE R860 Critical Care Ventilator.
GE has partnered with Ford to mass-produce a ventilator based on a design from Airon Corporation.
2.1.3 Medtronic
Medtronic is a Minneapolis-based global entity selling medical devices, amongst others also ventilators in 150 countries.
Top Medtronic ventilator products are the Puritan Bennett™ 980 Ventilator, Puritan Bennett™ 840 Ventilator, Newport™ e360 Ventilator, Newport™ HT70 ventilator Plus, Puritan Bennett™ (PB) 560.
Medtronic probably had one of the most remarkable reactions to the COVID-19 crisis. End of March 2020 Medtronic made the full design specifications produce manuals, design documents and, in the future, software code for its Puritan Bennett (PB) 560 portable ventilator hardware publicly available.
According to Medtronic, The PB 560 ventilator has a number of advantages, one being that it’s a relatively compact and lightweight piece of equipment that can be easily moved around and installed for use in a range of different healthcare environments and settings. In addition, this ventilating device which was first put on the market in 2010 has a decade of qualified, safe medical use in treating patients.
2.1.4 Royal Philips
Royal Philips is a global leader in health technology and a leader in manufacturing ventilators.
Ventilator products from Royal Philips are amongst others the Respironics V680, DreamStation BiPAP AVAPS and Trilogy Evo, the portable hospital-to-home ventilator.
Philips announced the end of March 2020 that in a response to the COVID-19 crisis it was increasing the production of certain critical care products and solutions to help diagnose and treat patients with the new coronavirus disease. In particular, Philips is ramping up the production of hospital ventilators and plans to double production within the next eight weeks and achieve a four-fold increase by the third quarter of 2020.
2.1.5 Smiths Medical
Smiths is the manufacturer of the PneupacTM paraPAC plus series of ventilators.
Smiths’ said it is working closely with the Ventilator Challenge UK Consortium to expand its supply chain capability, including its operations in Luton and numerous UK technology and engineering companies across automotive, aerospace, industrial and F1. The company is also in discussions with potential global partners to make its ventilator technology available to support the global fight against COVID-19.
2.2 Unconventional Ventilator Manufacturers
Unprecedented times call for unprecedented measures. Unconventional ways to stem the ventilator shortage turning to war-time solutions and companies like GM, Dyson and Rolls Royce to start producing the essential breathing equipment.
2.2.1 Dyson
Dyson, a British manufacturer of vacuum cleaners and other household appliances, has deployed its infrastructure and technology know-how to develop a medical ventilator that they coined CoVent. The famous household appliance manufacturer plans to fill a major order for the new device from the British National Health Service (NHS).
Dyson has pledged to produce 10,000 of its new CoVent machines for the British National Health Service (NHS) and will thereafter be able to export the device to other countries across the globe.
2.2.2 GE Health and Ford
Ford and GE Healthcare have licensed a ventilator design from Airon Corporation and plan to produce as many as 50,000 of them at a Michigan factory by July 2020 as part of a broader initiative to provide critical breathing equipment to help patients with severe COVID-19 infection to breathe.
Ford will inject manpower to help propel production at Airon’s Florida facility, where it produces just three of its Airon Model A ventilators per day. Further Ford will repurpose its Rawsonville Components Plant in Ypsilanti, Michigan for large-scale production of the Airon Model A-E ventilator. Ford has suspended production of its vehicles during the COVID-19 pandemic.
2.2.3 General Motors and Ventec Life Systems
Ventec Life Systems and GM have partnered to contribute to the production of critical care ventilators in response to the COVID-19 pandemic.
“This unique partnership combines Ventec’s respiratory care expertise with GM’s manufacturing might to produce sophisticated and high-quality critical care ventilators,” said Chris Kiple, CEO of Ventec Life Systems. “This pandemic is unprecedented and so is this response, with incredible support from GM and their suppliers. Healthcare professionals on the front lines deserve the best tools to treat patients and precision critical care ventilators like VOCSN are what is necessary to save lives.”
Ventec and GM are working around the clock to meet the urgent need for more ventilators. Efforts to set up tooling and manufacturing capacity at the GM Kokomo facility are already underway to produce Ventec’s critical care ventilator, VOCSN. Depending on the needs of the federal government, Ventec and GM are poised to deliver the first ventilators next month and ramp-up to a manufacturing capacity of more than 10 000 critical care ventilators per month with the infrastructure and capability to scale further.
2.2.4 Ventilator Challenge UK Consortium
Ventilator Challenge UK is a consortium of aerospace, automotive, consulting, software and engineering companies pulling forces to rapidly-produce ventilator devices based on models already in use in the UK. The UK government awarded the consortium with an order of 10,000 units.
Companies involved in the ventilator challenge: Airbus, BAE Systems, Ford Motor Company, GKN Aerospace, High-Value Manufacturing Catapult, Inspiration Healthcare Group, Meggitt, Penlon, Renishaw, Rolls-Royce, Siemens Healthineers and Siemens UK, Smiths Group, Thales, Ultra Electronics, Unilever, Accenture, Arrow Electronics, Dell Technologies, Microsoft and PTC.
UK-based F1 teams: Haas F1 McLaren, Mercedes, Red Bull Racing, Racing Point, Renault Sport Racing and Williams.
3 Ventilators for Developing Countries
3.1 COVID-19 In Developing Countries
The impact of COVID-19 on developing countries is expected to be significantly worse compared to most developed countries currently facing the same threat. It is not only the impact on the health crisis in the short term but also the long term social and economic effects this virus will have.
This is mainly due to poor living conditions and overpopulation that makes it difficult to maintain social distancing. Many citizens lack access to basic resources such as water which prevents proper hygiene. Poor energy infrastructure means many countries don’t have a continuous power supply to hospitals. Also limiting the country’s ability to respond is a drastic lack of resources.
The United Nations Development Program (UNDP) has launched a COVID-19 rapid response facility to assist the most vulnerable countries with responding to this threat. (United Nations Development Programme, 2020)
Most developing countries are heavily reliant on foreign exchange to generate income through exports and tourism. None of this is currently happening, in addition, investors are moving towards richer countries to protect their assets from the financial crisis caused by COVID-19. This makes it significantly more difficult for countries to gain access to additional finance to support the country in these trying times. (Hausmann, 2020).
The high prevalence of HIV infection and HIV-tuberculosis co-infection in especially African developing countries make the population as a whole more vulnerable and susceptible to develop acute breathing problems when infected with the COVID-19 virus. Initial data indicates that the mortality rate for individuals with certain chronic medical conditions is up to five times higher than the overall rate.
The weak health-care systems in general in developing countries will be highly challenged to provide the necessary care to COVID-19 patients. Many health-care systems in the developed world are swamped with severe cases and they have significantly fewer hospital beds per capita. For example, the most recent data available from the world bank indicates that Ethiopia has 0.3 beds per 1,000 people, India 0.7 per 1,000 people, Mali 01.1 per 1,000 people compared to an average of 5.6 in Europe. All in all, the developing countries have restrained resources including critical care units and most significant with this pandemic, ventilators.
Poor and limited medical facilities prevent countries from treating patients effectively and rely heavily on patients to be disciplined and self-isolate. This makes it very difficult to flatten the rate of infection. Especially if a patient is living with 6 other people. Someone still needs to go out and buy food despite the risk of spreading the infection.
It may therefore take much longer for developing countries to handle the crisis which could result in thousands of patients requiring medical care. With limited resources, it is unlikely that these countries will be able to afford expensive medical equipment such as ventilators to treat patients. More cost-effective solutions are crucial to help these countries overcome this threat.
The Africa Centres for Disease Control and Prevention, meanwhile, said this week that countries in the continent were looking to engage with wealthier countries to secure key supplies, including ventilators if the situation deteriorates.
3.2 Ventilator Requirements For Developing Countries
The high cost of a ventilator is not the only factor that needs to be considered when considering the provision of ventilators in developing countries to address the shortage.
In addition to the capital to actually buy even a basic ventilator the following is involved and required to be able to operate a typical ventilator:
– A small operation is required in the case of IV ventilation to insert the tube into the patient’s trachea;
– Adequate sedation of a patient;
– Electricity to power the ventilator
– Compressed oxygen is required for the ventilator;
– Sufficient personnel to monitor and wean patient of the ventilation once recovered;
– Resources of personnel and money to maintain the ventilator;
Second, ventilators can be sensitive machines and require appropriate maintenance.
In many countries, electricity and compressed oxygen is a scarce resource and must be available throughout a region before an institution will consider mechanical ventilation.
This indicates that in addition to the ventilator, management systems must be put in place to address all the requirements to put a patient on a ventilator and enable effective recovery.
This, however, presents a big opportunity to change the current approach in how ventilators are manufactured to make it fitter for the purpose for countries which may lack some of the resources required for a modern ventilator.
The requirements of a ventilator in a developing country is, therefore, a cheap, portable, easily deployable, easy to use and rechargeable battery-powered ventilator. (Krishnamoorthy, 2014)
3.3 Low-tech Ventilator Manufacturers
The global COVID-19 pandemic sparked innovation world-wide. A few manufacturers were already trying to address the need to produce a low-cost ventilator to fill gaps in hospitals not adequately furnished with high-end ventilators for the critically ill. The question remains though whether any of these solutions really have what it takes to be mass-produced in countries with poor infrastructure and non-existent supply chains. The real stress-test will be when one tries to mass-produce the designs in developing countries where there is no hobby shops, no electricity and no 3d printers available.
Here are four examples of the latest low-cost developments.
3.3.1 OneBreath
one breath is a company founded by physicians and engineers from Stanford University. They have a working prototype of a low-cost ventilator that they specifically designed to assist in circumstances like this pandemic.
Unfortunately, they are not producing yet and are not in a position to address the current need mainly due to a lack of funding. The manufacturing facility is also located in China which was only recently released from lockdown after the COVID-19 virus stopped production in the country.
You can read more about OneBreath here.
3.3.2 Nocca Robotics
Nocca Robotics as an Indian start-up barely two years old. Nocca develops water-less robots that clean solar plants.
The invasive ventilator being developed by the engineers at Nocca Robotics will cost 50 000 rupees ($662). Within five days of beginning work, a group of seven engineers at the start-up have three prototypes of a portable machine ready. They are being tested on artificial lungs, a prosthetic device that provides oxygen and removes carbon dioxide from the blood. By 7 April, they plan to be ready with machines that can be tested on patients after approvals.
See a video of the Nocca Engineers at work here.
3.3.3 The Breathing Project
A team of researchers and technicians from the Philipps University of Marburg and the University Hospital Gießen and Marburg (UKGM) has developed two different concepts for simple ventilators in a very short time. The devices can be manufactured quickly and inexpensively.
As a first approach, they developed a component for CPAP machines, so that they can be used as ventilators in intensive care units. Such machines are widely used and available e.g. in Europe since they are used to treat sleep apnoea. These modified CPAP machines will most probably not be robust enough for the treatment of severely infected COVID-19 patients but could be utilised once the patient has stabilised.
For developing countries, the team is currently developing simple devices based on so-called “Ambu Bags” as a second approach. These “Ambu Bags” or resuscitation bags are used in first aid and are available at low cost and in large quantities, where supply chains are still functioning efficiently. They consist of a mask that is pressed onto the face and a compressible bag that is compressed by hand at regular intervals for ventilation. The team is now developing mechanical devices that compress the bags periodically.
The team has the objective to make all technical information and construction instructions publicly available. You can find more information here.
3.3.4 Rice University
Rice University engineers have built a prototype of a ventilator using 3-D printed parts and hobby-store materials for possible use by coronavirus patients in dire need of ventilation.
The invention could help to help fill gaps in hospitals with an inadequate number of ventilators to serve the severely ill. The ventilator going by the name of ApolloBVM weighs 8 pounds and could be mass-produced for less than $200 each according to Rohith Malya, an emergency medicine doctor at Baylor College of Medicine and adjunct assistant professor of bioengineering at Rice, a Houston research university. You can read more about ApolloBVM here.
4 EPCM’s Contribution Towards The Ventilator Race
4.1 Rapid Development For Africa and Beyond
In our fight against the COVID-19 virus and determination to protect humanity, our team of multi-disciplinary engineers has invested in the rapid development of a low-cost fit-for-purpose ventilator system to support the increasing supply shortages facing Africa and beyond.
The design objective was to develop a fit-for-purpose product utilising readily available materials whilst complying with Medicines & Healthcare products Regulatory Agency (MHRA) design specifications and the South African Health Products Regulatory Agency’s (SAHPRA) regulations.
These ventilators will be produced from our workshops located in Pretoria, Secunda, Durban and Richards Bay, South Africa. EPCM, as an essential supplier and critical repair on essential services provided, also has the necessary permits to operate during COVID19 lockdown period/s.
4.2 Technical Design of the EPCM Ventilator
The low-cost ventilator is based on the technical design discussed in this section. Figure 3 illustrates the basic design.

Figure 3: EPCM Basic Design.
4.2.1 Key Parameters
The ventilator is designed to be controlled in accordance with the following key parameters:
- BPM (breaths per minute) between 8-30 BPM
- Tidal Volume (air volume pushed into lungs) 200-800mL
- I/E Ratio (inspiratory/expiration time ratio) 1:1 to 1:4
- Maximum pressure less than 40cm H2O
- PEEP of 5-15 cm H2O
- Manual override
- HEPA filtration when exhaling to avoid COVID-19 spreading
Airway pressure parameters:
- Maximum pressure: limited to 40 cm H2O at any time;
- Plateau pressure: limited to max 30 cm H2O
- The use of a passive mechanical blow-off valve fixed at 40 cm H2O
- Readings of plateau pressure and PEEP
- PEEP of 5-15 cm H2O
4.2.2 Essential Features
The essential features to ensure it is fit for purpose area:
- AC powered motor with battery backup: An electrical motor (AC) connected to a cam device is used for bag actuation. The volume and pressure are manually set on the control box to compress at the required frequency. The battery backup will be able to power the ventilator for a minimum of 60 minutes in case of a power failure.
- In-line filters: Because COVID19 can get aerosolised (airborne) and to protect clinical staff from certain infections, in-line filters between the ventilator unit and the patient (at the end of the endotracheal tube) are included.
- Heat and moisture exchanger: A heat and moisture exchanger are used in-line with the breathing circuit. An alarm trip in the case of failure conditions.
4.2.3 Ventilator Components
The number of components was kept to a minimum to ensure simplicity and ease of assembly for the end-user. It is made up of the following components.
- Support
a. Motor supports
b. AMBU bag support
c. Baseplate
d. Motor bracket - Electrical stepper ac motor with battery back-up
- AMBU Bag
- Control Unit
- Ventilation arm
- In-line filter
- Heat and moisture exchanger
4.3 EPCM Manufacturing Capabilities for Ventilators
EPCM has been part of multiple large design and construction projects across Africa. Five workshops in South Africa is utilised for all in-house manufacturing with strategic partners in our network to source any parts we are not able to manufacture in-house.
In addition, the company has the following ISO accreditations:
ISO 9001: 2015
ISO 14001: 2015
ISO 45001: 2018
ISO 3834
4.4 EPCM Offering
Based on the market and different requirements EPCM have developed three offerings to suit our potential clients. The following three offerings are available:
4.4.1 Design of Ventilation System Ready for Fabrication
This offer is aimed at clients who have resources available and want to manufacture the device locally. The design will also be supplied along with an operating manual. The benefits of the offer are local job creation and could potentially fast track manufacturing and delivery depending on the availability of parts and manpower.
4.4.2 Design and DIY Kit
This offer allows a user to self-assemble the unit on-site or at a local facility for distribution to medical facilities across the country. This offer has the benefit of saving transportation cost, more units can be delivered at a time and have a faster delivery time. EPCM also has the required network to fast-track the procurement of the required parts.
4.4.3 COMPLETE VENTILATOR UNIT
This option delivers a completed unit to the facility including an operating manual for the end-user. EPCM will again utilise its network to fast-track procurement and utilise our existing and available facilities for the manufacturing of the units.
5 Good To Know
5.1 How Our Lungs Work
Hackaday posted this informative article which summarises some basic principles to better understand lung function and the role ventilators play for those who cannot breathe naturally due to an illness. The article explains that our lungs take air in and pass it through smaller and smaller structures found in our lungs which allow tiny veins to absorb O2. CO2, in turn, is released from our lungs and we exhale it back into the atmosphere.
The most important driving force for the exchange of O2 and CO2 is the pressure difference (or concentration difference) between the O2 in the air and O2 in the blood. The same is true for CO2. The amount of O2 your body can absorb at a time is directly related to the amount of surface area available for the O2 to be absorbed into your blood.
By exhaling the CO2 in your lungs, you enrich the air with oxygen by inhaling which increases the rate at which O2 can be absorbed into your blood.
The surface area is therefore important for effective breathing. If any damage or blockages occur to the small structures required for the O2 exchange your body will receive too little O2 and won’t be able to function normally. Similar occurs for too much CO2 in your body. Too little oxygen and/or too much CO2 can result in damage to your organs as well as your brain.
5.2 What COVID-19 Does To Our Breathing
There are various factors that could reduce the surface area in our lungs or our ability to absorb O2 and release excess CO2. E.g. branches can be blocked by too much mucus and lung capillaries can become stiff or scarred (due to smoking). Any of these then cause a reduction in effectively absorbing O2 and have adverse effects on normal functioning. In extreme cases, this can lead to death.
COVID-19 can cause the following: pneumonia which is a lung infection causing the lungs to fill with fluid, dyspnea which is difficulty breathing and acute respiratory distress syndrome (ARDS). The George Washington University Hospital made an informative video to visually explain and show what COVID-19 does to a person’s lung as the virus spreads and infects a person.
Pneumonia causes excess mucus production and filling the structures in the lungs with liquid preventing effective absorption of O2. The lungs become inflamed and swell in an effort to get rid of the virus. All of these factors lead to ineffective breathing and can also cause scarring on the lung structures which constrict the structure, further reducing the surface area available for O2 exchange.
In severe cases, the only way to allow a patient to absorb enough O2 a release excess CO2 to enable normal organ functionality is through the use of a ventilator. In the case of COVID-19. Approximately 5 % of people that become infected will become critical and require ventilation to allow the person to survive. (Pathak, 2020)
Any scar tissue that is formed or constriction that occurs in the lungs due to COVID-19 or any respiratory disease is mostly unrepairable leading to lasting effects of the virus or illness. (Staff, 2020)
In short, the COVID-19 virus in severe cases prevents the patient from breathing normally to the extent that can lead to organ failure etc. due to a lack of oxygen in the blood. To prevent this a patient requires assistance with breathing and that is where the ventilator plays its critical role.
5.3 What is a Ventilator?
A patient suffering from COVID-19 in a severe condition cannot breathe normally and requires artificial respiratory assistance. A ventilator is a specially designed pump that aids respiration by delivering oxygenated air and permitting CO2 to escape from the lungs.
The task of assisting individuals with their breathing is highly delicate. For this, the utmost reliability and safety of the equipment are critical. Ventilators are mainly used in emergency medicine, intensive care medicine, anesthesiology, and home care. It offers mechanical ventilation to a patient when the patient cannot ventilate sufficiently without external assistance.
5.4 HIGH-LEVEL TECHNICAL SPECIFICATIONS OF A VENTILATOR
Figure 1 shows the main components of a ventilator:
- Power source: Electricity, gas or pressure generator
- Control Unit
- Blender (if not pure room air is provided)
- Valves
- Flow regulator
- Monitoring
- Sensors
- Safety Features
- Filters
- Alarms
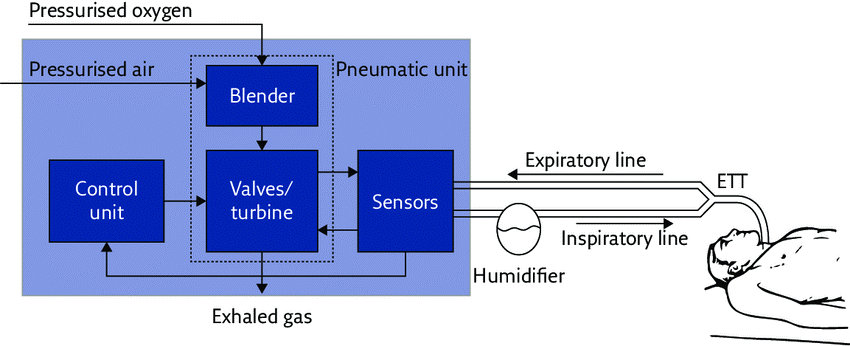
Figure 1: Basic components of the ventilator (Dellacà, 2017).
As technology has developed it has moved from a simple syringe-type system with air being forced into and out of the lungs to a highly automated ventilation system requiring no human input and alerting health personnel is anything is out of place.
5.4 History of the Ventilator
In the late 19th century the first versions of what we call a ventilator today were developed. In 1864 Alfred Jones invented a body enclosing device covering a person’s entire body. The enclosure’s pressure was changed which forced inhalation and exhalation. The design was improved mainly to help to drown survivors to start breathing again. The development was slow until 1929 when the polio epidemic occurred (Slutsky, 2015). In 1953 Dr Bjørn Ibsen in Copenhagen was the first doctor to formally use a ventilator in modern medicine. This helped to save numerous lives who would otherwise have died due to polio causing respiratory failure. The mortality rate was reduced from 87 % to 25 %. (CRONQVIST, 2003). See a video about the iron lung and the use of it during the polio epidemic at Boston Children’s hospital.
Since then the technology has developed significantly and is now a cornerstone of critical care medicine in the world. Various challenges were faced along the years. In 1997 it was found that mechanical ventilation could cause damage to the lungs which was referred to as biotrauma. As a result, measures and strategies were put in place to prevent this from causing severe damage and limiting the after-effects this could have on a person’s life. (Slutsky, 2015)
It is now a lifesaving intervention mainly due to excessive research and development. Although some of the dangers related to this life-saving device is still to be addressed more fully. The current ventilators have however leapt ahead of the first machine built-in 1864.
The availability of ventilators across the world however is not as it should be. Most developed countries have enough resources to acquire ventilators. In developing countries, the availability of ventilators is limited mainly due to the high cost and restricted resources of the medical facilities. This means many people don’t have access to a ventilator when they really need it.
We have come quite a long way. Figure 2 shows the progress of the ventilator.

Figure 2: Development in the ventilator. (Slutsky, 2015), (Yartsev, 2019)
5.5 When is a Ventilator a Necessity?
A patient will be placed on a ventilator when they show signs of respiratory failure. There are numerous reasons a patient may be placed on a ventilator. Specifically, in the case of the COVID-19 outbreak patients are placed on a ventilator when they experience breathing difficulties which could place their lives in danger.
According to Healthline symptoms of respiratory failure are: difficulty breathing or shortness of breath, coughing up mucus, wheezing, rapid breathing, fatigue, anxiety, confusion and daily headache. (Healthline, 2020)
Before going on a mechanical there may be other attempts to increase a patient’s oxygen levels. These “non-invasive” methods of ventilation can include masks and oxygen tanks.
With COVID-19, medical staff tend to avoid non-invasive methods because patients would cough and splutter which in turn increases the risk of the virus being transferred to medical staff.
5.6 Type of Ventilators
Two types of ventilators are typically used in modern medicine: invasive and non-invasive.
Non-invasive (NIV) entails a mask being placed on a patient that is connected to a ventilator that then pushes air into the lungs and allows the patient to exhale again releasing air into the atmosphere. This video demonstrates how non-invasive ventilation works.
Invasive (IV) requires a pipe to be inserted into the throat. This video shows how this is usually done through a process called intubation.
NIV was developed after IV but is preferred in numerous cases due to complications involved in the intubation if IV and standard mechanical ventilation.
Patients have also been found to recover quicker after NIV compared to IV. (Brochard, 2003)
5.7 Ventilator Breath Types
Each type of ventilator can assist a patient to breathe in one of three ways. Controlled Breaths is where the ventilator completely control’s a patient’s breathing. This is only done in cases where a patient is paralysed or doesn’t have a respiratory drive (e.g. under sedation). Secondly, a ventilator can give assisted breaths. This means the ventilator will deliver breaths if the patients show an indication of taking a breath or if after 5 seconds the patient hasn’t tried to breathe it will deliver a breath. Lastly, a ventilator can give supportive breaths which basically means once a patient tries to take a breath the ventilator will assist but will not give a full breath. This is only to support a patient not breath for them. (Lodeserto, 2018)
This article explains the concept quite well.
5.8 Modes of Ventilation
In addition to the types and type of breath that a ventilator can deliver. There are also different modes of mechanical ventilation. The mode that will be selected is selected by the medical practitioner based on the specific patient requirements.
The two main types are pressure or volume control. Modern categories distinguish between the trigger (flow or pressure), limit (size of breath) and cycle (end of breath).
If you are interested in learning about all the different modes. This article is quite a good start to understanding the different modes.
For volume modes, there are assist-control ventilation and synchronized intermittent-mandatory ventilation. With regards to pressure modes, there is pressure-controlled ventilation, pressure support ventilation, pressure-controlled inverse ratio ventilation and airway pressure release ventilation.
As technology has developed there are also dual modes such as pressure regulated volume control mode and interactive modes such as proportional assist ventilation. (Open Anesthesia, 2020)
About EPCM
6 References
Megan L. Ranney, M. M. (2020, 03 25). Critical Supply Shortages — The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. Retrieved from The New England Journal of Medicine: https://www.nejm.org/doi/full/10.1056/NEJMp2006141
Pathak, N. (2020, 03 25). What does Covid-19 Do To Your Lungs? Retrieved from WebMD: https://www.webmd.com/lung/what-does-covid-do-to-your-lungs#2
Staff, M. C. (2020, 03 31). Pulmonary Fibrosis. Retrieved from Mayo Clinic: https://www.mayoclinic.org/diseases-conditions/pulmonary-fibrosis/symptoms-causes/syc-20353690
Dellacà, R. &. (2017). rends in mechanical ventilation: Are we ventilating our patients in the best possible way? Breathe, 84-98.
CRONQVIST, P. G. (2003). The first intensive care unit in the world: Copenhagen 1953. Acta Anaesthesiol Scand, 1190-1195.
Slutsky, A. (2015). History of Mechanical Ventilation. From Vesalius to Ventilator-induced Lung Injury. American Journal of Respiratory and Critical Care Medicine.
Yartsev, A. (2019, 06 20). Breathing circuits for manual and mechanical ventilation. Retrieved from Deranged Physiology: https://derangedphysiology.com/main/cicm-primary-exam/required-reading/respiratory-system/Chapter%20505/breathing-circuits-manual-and-mechanical-ventilation
Healthline. (2020, 03 25). Healthline. Retrieved from Chronic Respiratory Failure: https://www.healthline.com/health/chronic-respiratory-failure#diagnosis
Brochard, L. (2003). Mechanical ventilation: invasive versus noninvasive. European Respiratory Journal, 31s-37s.
Lodeserto, F. (2018, 03 18). Simplifying Mechanical Ventilation – Part I: Types of Breaths. Retrieved from RebelEM: https://rebelem.com/simplifying-mechanical-ventilation-part/
Open Anesthesia. (2020). Modes of Mechanical Ventilation. Retrieved from Open Anesthesia: https://www.openanesthesia.org/modes_of_mechanical_ventilation/
Nations, U. (2019). World Population Aging. New York: United Nations.
United Nations Development Programme. (2020). COVID-19: The looming crisis in developing countries threatens to devastate economies and ramp up inequality. United Nations Development Programme.
Hausmann, R. (2020, 03 24). Flattening the COVID-19 Curve in Developing Countries. Retrieved from World Economic Forum: https://www.weforum.org/agenda/2020/03/flattening-the-covid-19-curve-in-developing-countries/
Krishnamoorthy, V. V. (2014). The need for ventilators in the developing world: An opportunity to improve care and save lives. Journal of global health.
al., F. Z. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. China: Lancet.
Fei Zhou, T. Y. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet, 1054-1062.

 To all knowledge
To all knowledge