We look at the Anaerobic Digester Design in South Africa. South Africa’s electricity is produced mainly from coal because it is the most abundant source of energy. It is the most widely used primary source of fuel and contributes to about 77% of the country’s primary energy needs. Coal contributes to greenhouse gas emissions to the atmosphere that leads to global warming.
Fossil fuels contribute to the increase in the concentration of carbon dioxide in the atmosphere, hence alternative energy sources (renewable energy) must be used in the place of fossil fuels. The commercial production of biogas and another alternative renewable energy source such as solar energy, wind energy, hydropower, geothermal will definitely give a drive for the development of the economy.
Energy derived from biogas is used in the form of fuel, heat, and electricity. Biogas is a renewable source of energy derived from biodegradable substrates such as agricultural wastes, animal wastes, domestic wastes, crops and industrial waste. It is produced by anaerobic digestion, which is a biochemical process in the absence of oxygen. The main product of biogas is methane and carbon dioxide.
Anaerobic digestion is often considered to be a complex process, the digestion itself is based on a reduction process consisting of a number of biochemical reactions taking place under anoxic conditions. Methane formation in anaerobic digestion involves four different steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis.
Generally in an anaerobic digestion process, the rate-limiting step can be defined as the step that causes process failure under imposed kinetic stress. In other words, in a context of continuous culture, kinetic stress is defined as the imposition of a constantly reducing value of the solids retention time until it is lower than the limiting value; hence it will result in a washout of the microorganism.
Most researchers report that the rate-limiting step for the complex organic substrate is the hydrolysis step due to the formation of toxic byproducts (complex heterocyclic compounds) or non-desirable volatile fatty acids (VFA) formed during hydrolysis: whereas methanogenesis is the rate-limiting step for easy biodegradable substrates.
The anaerobic digestion process can be divided into two phases as illustrated in Figure 1. The microorganism carrying out the degradation reactions in each of these phases differ widely regarding physiology, nutritional needs, growth kinetics, and sensitivity to the environment. Very often, it is difficult to keep a delicate balance between these two groups: the acid-forming and the methane forming microorganisms, which lead to reactor instability and consequently low methane yield. The two main groups of microorganisms could be physically separated with the intention of making use of the difference in their growth kinetics. In order to accomplish phase separation, several techniques have been employed such as membrane separation, kinetic control, and pH control.

Figure 1: Phase separation of the anaerobic digestion system
This is the first step in the anaerobic digestion process, it involves the enzyme-mediated transformation of insoluble organic materials and higher molecular mass compounds such as lipids, polysaccharides, proteins, fats, nucleic acid etc. into soluble organic materials i.e. to compounds suitable for the use as the source of energy and cell carbon such as monosaccharides, amino acids and other simple organic compounds. This step is carried out by strict anaerobes such as bactericides, clostridia and facultative bacteria such as streptococci etc. This first stage is very important because large organic molecules are simply too large to be directly absorbed and used by microorganisms as a substrate/food source. To accomplish biodegradation, certain microorganisms secrete different types of enzymes, called extracellular enzymes, which “cut” the larger molecules up into smaller pieces that the microorganism can then take into the cell and use as a source of energy and nutrition. Some microorganisms secrete several different enzymes, which allow them to break down different types of organic materials. Other microorganisms are specialised. For example, they secrete enzymes that break down either sugar or protein. Microorganisms that break down different sugars are called saccharolytic, while those that break down proteins are called proteolytic. There are different enzymes for sugars, proteins, fats etc. The rate of decomposition during the hydrolysis stage depends greatly on the nature of the substrate. The transformation of cellulose and hemicellulose generally takes place more slowly than the decomposition of proteins.
The monomers produced in the hydrolytic phase are taken up by different facultative and obligatory anaerobic bacteria and are degraded further into short-chain organic acids such as butyric acids, propanoic acids, acetic acids, alcohols, hydrogen and carbon dioxide. The concentration of hydrogen formed as an intermediate product in this stage influences the type of final product produced during the fermentation process. For example, if the partial pressure of the hydrogen were too high, it would decrease the number of reduced compounds. In general, during this phase, simple sugars, fatty acids and amino acids are converted into organic acids and alcohols.
The products produced in the acidogenic phase are consumed as substrates for the other microorganisms, active in the third phase. In the third phase, also called the acidogenic phase anaerobic oxidation is performed. Products which cannot be directly converted to methane by methanogenic bacteria are converted into methanogenic substrates, volatile fatty acids and alcohols (VFA) are oxidized into methanogenic substrates like acetate, hydrogen and carbon dioxide, VFA with carbon chains longer than one unit are oxidized into acetate and hydrogen. It is important that the organisms which carry out the anaerobic oxidation reactions collaborate with the next group, the methane forming microorganisms; this collaboration depends on the partial pressure of the hydrogen present in the system. Under anaerobic oxidation, protons are used as the final electron acceptors which lead to the production of H2. However, these oxidation reactions can only occur if the partial pressure of hydrogen is low, which explains why the collaboration with the methanogens is very important since they will continuously consume the H2, to produce methane. Hence during this symbiotic relationship inter-species hydrogen transfer occurs.
In the methanogenic phase, the production of methane and carbon dioxide from intermediate products is carried out by methanogenic bacterial under strictly anaerobic conditions. Methanogenesis is a critical step in the entire anaerobic digestion process as it is the slowest biochemical reaction of the process. Methanogenesis is the final stage whereby methanogens bacteria converts hydrogen, acetic acid, and carbon dioxide to methane and carbon dioxide. Equation 1 shows a simplified generic anaerobic digestion.
C6H12O6 ® 3CO2 + 3 CH4 (1)
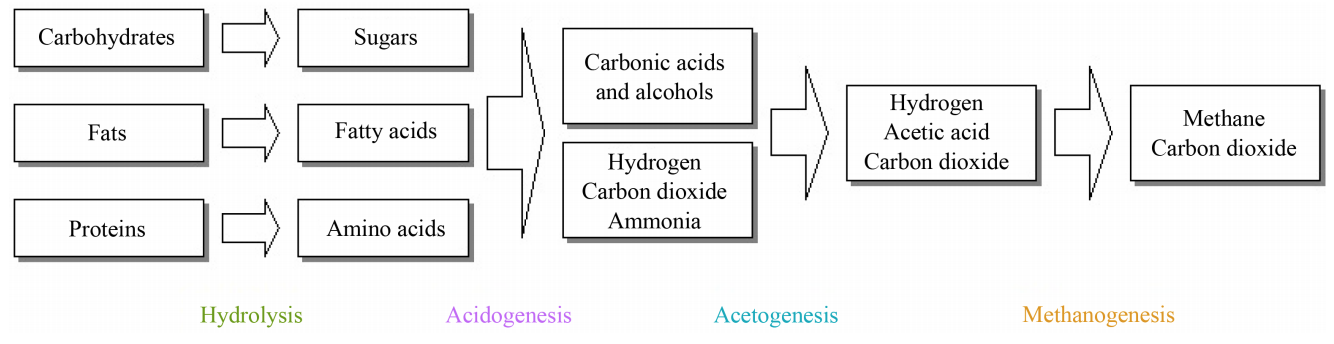
Figure 2 shows the whole biochemical process.
Figure 2: The key process stages of anaerobic digestion.
The activity of biogas production depends on various parameters that include: temperature, partial pressure, pH, hydraulic retention time, C/N (Carbon to Nitrogen) ratio, pre-treatment of feedstock, a trace of metals (trace elements) and concentration of substrate.
As a guideline, the C/N ration for anaerobic digestion should be around 25-30:1. Should more Carbon be present the biochemical decomposition slows down?
Batch or Continuous configuration
AD can be performed as a batch or a continuous process depending on the substrates being digested and the configuration of the digester. In a batch process, the substrate is added to the digester at the start of the process and sealed for the duration of the retention time (RT). After digestion, biogas is collected and the digester is partially emptied. They are not emptied completely to ensure inoculation of fresh substrate batch with bacteria from the previous batch.
In a continuous digestion process, organic matter is constantly added in stages to the digester on a daily basis. In this case, the end products are constantly removed resulting in constant biogas production. Single or multiple digesters in a sequence may be used.
The selection of biogas digester depends on the dry matter (DM) content of the digested substrate. There are two AD technologies systems: wet digestion which is liquid digestion; when the average DM content of the substrate is less than 15% and dry digestion which is solid digestion; when the DM content of the substrate is more than 15% (usually from 20 to 40%). Wet digestion is applied for substrates like manure and sewage sludge, while dry digestion is applied for solid municipal bio-waste, solid animal manure, high straw content, household waste, and green cuttings, grass from landscape maintenance or energy crops. Table 1 shows the characteristics of anaerobic digesters technologies while Table 2 shows the comparison of various digesters types.
Table 1: Main characteristics of Anaerobic Digester technologies

Table 2: Comparison of various Digester Types

Developing a biogas plant design is essentially the final stage of the planning process. However, it is mandatory for the designer to familiarize themselves with basic design considerations in advance. Ultimately, a successful plant design should be able to respond to quite a number of factors, and these include:
A. Climate
The design should respond to the prevailing climatic conditions of the location. Bearing in mind that biogas plants operate optimally at temperature ranges between 30°C to 40°C, in cooler regions, it is advisable for the designer to incorporate insulation and heating accessories to the design.
B. Substrate quality and quantity
The type and amount of substrate to be used on the plant will dictate the sizing of the digester as well as the inlet and outlet design.
C. Construction materials available
If the materials required for the plant set up can be sourced locally at affordable rates so as to maintain the plant set up costs within manageable ranges, then the design is preferred to that whose materials have to be imported.
D. Ground Conditions
Preliminary geotechnical investigations can guide the designer on the nature of the subsoil. In cases where the hardpan is a frequent occurrence, the design installation plan must be done in such a way that deep excavations are avoided because this would then increase the construction costs tremendously.
E. Skills and Labour
Biogas technology is sophisticated and hence requires high levels of specialized skilled labour. The labour factor cuts across from the planner to the constructor up to the user. However, gaps can be reduced through training of the involved parties at a cost.
F. Standardization
Prior to the commissioning of the design, the planner must carefully study the prevailing standards already on the market in terms of product quality and pricing, especially for large scale projects.
Several Decisions Support (DS) tools have been developed to give unbiased results when it comes to making decisions on technology selection. These include Multi-Criteria Decision Analysis (MCDA) techniques, the use of grey statistics and Technology Identification, Evaluation, and Selection (TIES) methods among others. In principle, all technology selection methods are based on the steps as summarised below;
MCDA is an approach employed by decision-makers to make recommendations from a set of finite seemingly similar options basing on how well they score against a predefined set of criteria. MCDA techniques aim to achieve a decisive goal from a set of alternatives using pre-set selection factors herein referred to as the criteria.
The selection criteria are assigned weights by the decision-maker basing on their level of importance. Then using appropriate techniques, the alternatives are awarded scores depending on how well they perform with regard to particular criteria. Finally, ranks of alternatives are computed as an aggregate sum of products of the alternatives with corresponding criteria. From the ranking, a decision is then made.
Multi-criteria decision analysis (MCDA) techniques can be successfully applied to choose a biogas digester technology from a list of potential alternatives for an anaerobic digestion (AD) system based on:
Using MCDA to analyse the various biodigester models presents a successful option owing to the fact that all the critical attributes are directly measurable and non-subjective.
A detailed feedstock analysis to determine the quantity and quality of the selected feedstock, which directly impacts the selected technology’s sizing needs to be completed. Using the MCDA technique, a suitable biogas model can be selected and from the substrate analysis, the appropriate size of the biogas digester can be determined.
The size of the digester, i.e. the digester volume Vd, is determined on the basis of the chosen retention time RT and the daily substrate input quantity Sd.
Vd = Sd x RT [m3 = m3/day x number of days] (2)
The retention time, in turn, is determined by the chosen/given digesting temperature. For an unheated biogas plant, the temperature prevailing in the digester can be assumed as 1-2 Kelvin above the soil temperature. The seasonal variation must be given due consideration, however, i.e. the digester must be sized for the least favourable season of the year. For a plant of simple design, the retention time should amount to at least 40 days. Practical experience shows that retention times of 60-80 days, or even 100 days or more, are no rarity when there is a shortage of substrate. On the other hand, extra-long retention times can increase the gas yield by as much as 40%.
The substrate input depends on how much water has to be added to the substrate in order to arrive at a desirable solids content (typically between 4 – 8% for wet digestion).
Substrate input (Sd) = biomass (B) + water (W) [m3/day] (3)
In most agricultural biogas plants, the mixing ratio for dung (cattle and / or pigs) and water (B:W) amounts to between 1:3 and 2:1. The ratio of B:W for the organic fraction of municipal solid waste (OFMSW) is in the region of 1:4.
The amount of biogas generated each day G [m3 gas/d], can only be estimated based on actual data recorded for the digester and substrate type and is calculated on the basis of the specific gas yield Gy of the substrate and the daily substrate input Sd.
The calculation can be based on:
The volatile solids content VS
G = VS × Gy(solids) [ m3/d = kg × m3/(d×kg) ] (4)
the weight of the moist mass B
G = B × Gy(moist mass) [ m3/d = kg × m3/(d×kg) ] (5)
The temperature dependency is given by:
Gy(T,RT) = mGy × f(T,RT) (6)
where
Gy(T,RT) = gas yield as a function of digester temperature and retention time
mGy = average specific gas yield, e.g. l/kg volatile solids content
f(T,RT) = multiplier for the gas yield as a function of digester temperature T and retention time RT
As a rule, it is advisable to calculate according to several different methods, since the available basic data are usually very imprecise, so that a higher degree of sizing certainty can be achieved by comparing and averaging the results.
The digester loading Ld is calculated from the daily total solids input TS/d or the daily volatile solids input VS/d and the digester volume Vd:
Ldt = TS/d ÷ Vd [kg / (m3 day)] (7)
and:
Ldv = VS/d ÷ Vd [kg / (m3 day)] (8)
Then, the calculated parameters should be checked against data from comparable plants in the region or from pertinent literature.
The size of the gasholder, i.e. the gasholder volume Vg, depends on the relative rates of gas generation and gas consumption. The gasholder must be designed to:
Vg1 = gcmax × tcmax = vcmax Vg2 = Gh × tzmax (9)
with
gcmax = maximum hourly gas consumption [m3/h]
tcmax = time of maximum consumption [h]
vcmax = maximum gas consumption [m3]
Gh = hourly gas production [m3/h] = G ÷ 24 h/d
tzmax = maximum zero-consumption time [h]
The larger Vg -value (Vg1 or Vg2) determines the size of the gasholder. A safety margin of 10-20% should be added:
Vg = 1.15 (±0.5) × max(Vg1,Vg2) (10)
Practical experience shows that 40-60% of the daily gas production normally has to be stored.
The ratio Vd ÷ Vg (digester volume ÷ gasholder volume) is a major factor with regard to the basic design of the biogas plant and ranges typically from 3:1 to 10:1 depending on the feedstock.
South Africa has experienced very limited market penetration for bio-digesters and biogas. In Germany, one of the leading European countries utilizing biogas, around 1000 plants are built every year. In India, more than 12 million plants, ranging from small domestic units to large commercial plants, are in operation. Even Uganda has more than double the estimated 300 biogas plants that South Africa are operating at present.
In South Africa the waste management landscape is changing rapidly since land around the larger metropoles for landfill use is becoming very limited and expensive. A rapid departure is needed from our current approach of only throwing away instead of recycling materials where possible and utilize the organic fraction of municipal solid waste (OFMSW) for biogas generation. South Africa has also ratified the Kyoto protocol and committed to reducing greenhouse gas (GHG) emissions by at least 34% by 2020, and a further 45% by 2030. The proposed Carbon Tax due for roll-out will also grease the wheels to promote more green technologies like biogas generation.
The market for gas in South Africa has been increasing steadily since the introduction of the natural gas from Mozambique to industries and in certain areas of Johannesburg even to residential customers. With the new technologies available to feasibly “clean up” the biogas by removing the trace elements of hydrogen sulphide and reduce the carbon dioxide it is possible to successfully store and pressurize the bio-methane. This enables not only the use of the biogas for electricity generation as in the past but also for utilization anywhere from heating, cooking and transportation fuels in the form of CNG or even LNG (Liquified Natural Gas).
EPCM Consultants has already monetized the only “natural” bio-gas reserves in South Africa by converting it to compressed natural gas (CNG) to run Harmony mining’s busses. EPCM is also currently developing the next step to liquefy the gas to increase the energy density and enable more opportunities for usage. More recently EPCM completed a design to convert a customer’s anaerobic digester to utilize the biogas generated from organic waste material as compressed natural gas (CNG), instead of generating electricity. They will be using the CNG to supplement their transportation fuel requirements increasing the value generation from their anaerobic digester.
Biogas as an almost previously untapped resource in South Africa presents huge opportunities for utilizing untapped renewable resources (from manure to organic wastes) to expand the gas market in areas not having access to the Sasol gas pipeline infrastructure by providing a cost-competitive clean energy source.
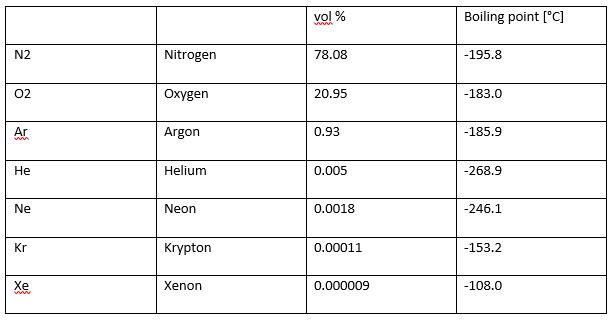
An Air Separation Unit (ASU) is a type of technology that separates air into its primary components. The most abundant are Nitrogen, followed by oxygen, and then argon, along with other inert gases in small amounts. The composition breakdown is summarised in Table 1 below.
Various methods or technologies can be used to separate air into its components. The oldest and most popular is cryogenic or fractional distillation. Other methods include pressure swing adsorption (PSA) and membrane technology, but hybrids of these technologies also exist, and new and improved methods are being researched. These three methods are explained in the sections that follow.
Table 1: Composition of Dry Air (Adapted from Linde Engineering, 2017)
Oxygen, constituting 21% of air by volume, is the most widely used air product with many industrial applications. The oxygen production from air separation is a large and growing industry, producing nearly 100 million tons of oxygen annually (Hashim, 2011), and demand is growing as future clean energy technologies increasingly rely on oxygen. While oxygen is the primary product, air separation methods also yield other air products with various by-products. These technologies vary in scale and application efficiency, as summarised in Table 2.
Table 2: Comparison of process alternatives for oxygen production from air separation (Adapted from Hashim, 2011).

For air to be separated into its different components, it must first be liquefied (liquefaction of air). Liquefaction occurs when a gas is subjected to temperatures and pressures below its critical point or conditions. The critical temperature of the air is -140.7°C (132.5 K), and the critical pressure is 37.7 bar. This means that air has to be cooled below this temperature to become a liquid for separation. This critical temperature varies depending on the pressure of the gas, in that a higher temperature is required for lower pressure. This relationship is proportional but not linear (Linde Engineering, 2017).
In 1895, Carl von Linde successfully liquefied air, attempting to obtain liquid carbon dioxide to be used in the brewing industry (Flavell-While, 2010). It was only later that separation methods were developed. This liquefaction process took three days. Separation methods have come a long way since then. Below is an early diagram of the liquefaction process.

Figure 1: Von Linde’s original drawing of his air liquefaction process (Flavell-While, 2010)
Cryogenic air separation is the most common and standard technology used for the separation of air into its constituents. It produces a high-purity product and is the most developed method to date (Hashim, 2011). This is a highly energy-intensive process due to the low temperatures that need to be achieved, as shown in Table 1 above.
The air is first filtered, compressed and then chilled to -185°C. This liquefies the air, and this liquid stream is sent to a distillation column to be separated. This uses the natural temperature gradient within the column to allow nitrogen to leave the top of the column as gas and oxygen as a liquid at the bottom. Argon can also be separated by taking a stream somewhere in the middle of the column where the argon concentration is the highest and feeding this into another column where nearly pure argon is separated from the other gases.
Some improvements to this process included using packed towers instead of traditional trayed towers, introducing heat integration, and using more efficient compressors to reduce energy consumption. Argon separation has also been improved. Conventionally, hydrogen catalytic converters were needed, but modern processes only require structured packings. This method is reaching its maturity level, and industries are looking at other air separation methods, which are continually being developed, as there is potential for high purity and lower energy consumption (Hashim, 2011).
Below are more detailed steps for a typical cryogenic air separation process (Linde Engineering, 2017). This process produces gaseous pure oxygen and nitrogen, as well as liquid oxygen, liquid nitrogen and liquid argon, with internal compression. A diagram for this process can be seen in Figure 2.

Figure 2: Typical Cryogenic Air Separation Process (Linde Engineering, © 2017)
The basis of pressure swing adsorption (PSA) is that when gases are put under high pressure, they are attracted to solid surfaces and hence adsorbed. This is a proportional relationship in that the higher the pressure, the more gas is adsorbed. The adsorbent bed is known as a zeolite.
Filtered air enters the PSA generator, which contains zeolites that absorb nitrogen and/or argon. This is done at high pressures, which cause the gases to be attracted to the solid surface of the zeolites. Purified oxygen can then be separated. Once the oxygen has been collected, the pressure then swings to low pressure to desorb the nitrogen, which can also be collected. As soon as the pressure is reduced again, the gas is desorbed (released). This additionally frees up the adsorbent and makes it ready for the next cycle.
Different gases can be adsorbed by using different solid particles based on what the gases are more easily attracted to, so this method typically has relatively high product purity. However, by-product purity is still being improved. If the zeolite attracts nitrogen, then some or all of the nitrogen will be adsorbed when a stream of air is pressurised and can be released after it is depressurised again. The exiting stream will, therefore, be richer in oxygen compared to the entering stream, and this oxygen will be collected. The zeolite can then be reused for another batch. When two adsorbent vessels are used, the process becomes nearly continuous (Ruthven, 1990), as illustrated in Figure 3 below.
This is the most suitable method for producing oxygen for small to medium-scale plants (20-100 tons/day). Large-scale plants typically use cryogenic separation (more than 100-300 tons/day). This is also an older technology; hence, it has been perfected over the years in terms of adsorbents used and energy consumption. This is the favoured alternative to traditional cryogenic distillation, as it has a significantly lower energy demand and a high product purity. There are various variations of PSA, including vacuum swing adsorption, temperature swing adsorption, vacuum-pressure swing adsorption, and many more. These systems rely on the zeolites to trap nitrogen, producing oxygen with a purity of 90% to 95%. Different adsorbents are being investigated continuously to improve this process even more. The performance of this process is highly dependent on the pressure, which can be more energy-intensive, as greater energy is required for greater pressure and hence greater performance (Hashim, 2011).
The original pressure swing air separation process uses a zeolite adsorbent that is selective to nitrogen and hence produces an oxygen-rich exit stream. This method is predominately in small-scale units, but many modifications have been made to adapt this method for large-scale units to reduce energy demand. Nitrogen can theoretically be recovered from the desorption of the zeolite, but this is not of very high purity, so it is more desirable to use a zeolite that is selective to oxygen. This, however, is still being perfected (Ruthven, 1990).
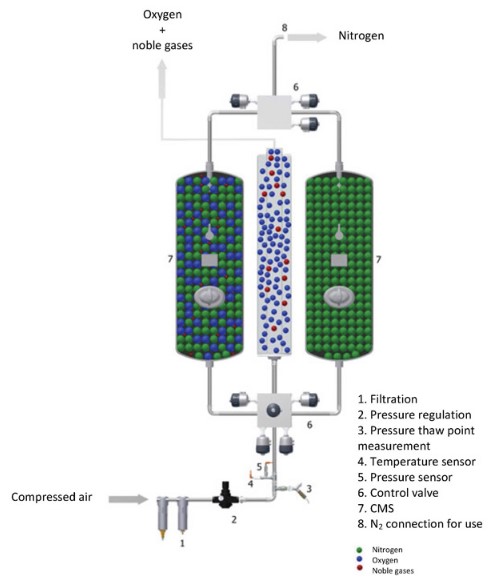
Figure 3: Pressure Swing Adsorption Technology (Inmatec, 2017)

Figure 4: Schematic Representation of the Oxygen Transport in Dense MIEC Ceramic Membrane (Hashim, 2011)
The third type of air separation is via membrane separation technologies. This modern technology is not yet well established but is potentially very promising. This is the most recent technology, and new developments are constantly emerging from it, such as new types of membranes and hybrid systems involving both cryogenic distillation and pressure swing systems. Currently, dense ceramic membranes are used. This technique can separate oxygen from the air, usually at high temperatures of 800-900°C. This method also has high by-product purity recovery, so it can be used to potentially separate any component of air (Hashim, 2011).
Traditionally, ceramic membranes with mixed ionic electronic conducting (MIEC) characteristics have been explored due to their potential to produce high-purity oxygen. This type of membrane does not require electrodes or an external circuit to operate. As illustrated in Figure 4 above, the oxygen partial pressure gradient creates an internal short circuit through electronic conductivity (Hashim, 2011).
This method separates oxygen from air by taking advantage of the oxygen partial pressure gradient. There is a high oxygen partial pressure side of the membrane on the feed side and a low one on the sweep side. The oxygen naturally permeates from the high-pressure side to the low-pressure side. The flux of electrons through the membrane keeps the overall charge neutral. As the gas stream flows along the membrane, it is depleted of oxygen through the permeation of the membrane via the pressure gradient, and the sweep stream becomes oxygen-enriched. This sweep stream flows counter currently to the air feed. This oxygen is then collected (Hashim, 2011).
This technology is still in its early stages and continues to be researched. It holds the potential to produce a very high-quality product, with separation possible at high temperatures without the need for liquefaction. Although not yet widely used, it is predicted to become the dominant method in the industry in the future (Hashim, 2011).
References:
Bibliography:
The future of natural gas and liquid natural gas (LNG) looks promising in Africa as a natural gas resource and a potential import market. African governments are eyeing clean energy as a bridge to rapid economic growth. However, the key stumbling block has been its transportation options. Nevertheless, natural gas liquefaction now opens the possibility of better transportation logistics. This article is a summarized report of the research documented by the Department of Energy in the Office of International Affairs in the U.S. It aims to explore the feasibility and understanding of Natural Gas and LNG projects in Africa.
EPCM has categorized this summary into five main sections:
Natural gas marketing can be viewed from two perspectives: the local market and the global market.
The first overseas gas trade occurred in 1959 between the United States and the United Kingdom. Since then, regions have been teaming up depending on their proximity to reduce LNG transportation costs. Today, the global gas market comprises three primary regions: Europe, the Asia-Pacific (currently the largest market), and North America (Atlantic Basin). Japan, South Korea, and Taiwan are the world’s leading importers of LNG, with India and China slowly catching up.
While there is more than enough demand for natural gas, oversupply has been a threat in the past. However, as more uses of natural gas are discovered each day, a shortage in its supply is also possible. In 2016, 258 million metric tonnes of LNG were traded globally. Nineteen countries exported LNG, while 34 countries imported it.
The above factors have led to an oversupply of LNG in the global market, resulting in a significant price drop. This situation could persist as a short- to medium-term crisis. Before planning new projects, new gas investors should carefully forecast when market rebalancing may occur. The key is to have sales above the marginal cost of production. Commercial and financial pressures may drive a short-term realignment of the LNG market; however, if this correction does not occur, oversupply could extend well into the next decade.
The domestic market has a solid demand for compressed natural gas (CNG) and its by-products, such as petroleum liquids. Transportation is also cost-effective where pipeline transmission can be used. The host’s national gas company markets the gas locally and develops the necessary infrastructure.
In Africa, domestic and regional pipelines have expanded the local market, and manufacturing industries continue to drive demand for CNG as a feedstock. Additionally, CNG is a favoured fuel source for power generation and gas-powered vehicles. However, infrastructure and market development remain limited.
Domestic markets have more demand for natural gas as a fuel for power production. For investors, proper planning is critical to ascertain reliability. Cost-effectiveness and safety of the end product (both to the users and the environment) should also be considered. Identify demand centres (bearing in mind future expansions). Then, decide whether to build these power plants closer to the gas source or the demand centres. A few factors to consider include;
Here is a link for more information on how natural gas fuels power generation.
A potential natural gas customer (vehicle, power plant, or ship) has to be convinced that this gas will always be available. The gas must also outweigh their current options and the risk associated with change.
Sub-Saharan African countries have the potential to become major natural gas producers. Gas reserves are being explored daily. Tanzania and Mozambique recently discovered gas reserves of over 250 trillion cubic feet (TCF).
Most African nations also have a potential LNG import market due to:
According to the September 2016 report generated by the U.S. Power Africa Roadmap, one of the Vision 2030 goals in sub-Saharan Africa is to increase power generation by over 30,000 megawatts (MW). Assuming natural gas is their preferred energy source, this translates to over 42 MTPA of liquid natural gas. In addition, the African Development Bank Group, via the Energy for Africa project, aims to make electricity available to all nations by 2025.
In Africa, natural gas prices vary. They are established by the seller after some negotiation with the buyers. The prices range between $1.21/MMBtu and $8.4/MMBtu (a case study in Mozambique, Ghana, and Nigeria). The market demand is so good that most African gas producers focus on the domestic market. Nigeria is a good example.
Pipelines face significant challenges in offering flexibility, especially in mountainous regions or areas with insecure borders, where piped gas supply may not be feasible. In such cases, compressed natural gas becomes their plan B. However, pipeline supplies still pose a threat to LNG markets. Primary causes of market uncertainties include:
These factors often lead shippers to sell cargoes initially reserved for long-term gas contracts. This approach assists in offsetting the initial costs of infrastructure and LNG carriers. However, these conditions negatively impact the efficient transportation and marketing of LNG.
A typical natural gas project is capital-intensive and can last between 20 and 40 years. Investors must allocate risk and define the functions of each participant. This will ascertain sufficient returns on investment and the ability to pay off debts. Consider the possibility of future expansion as well. Poorly structured projects scare off potential buyers due to the high risk involved.
The most expensive area in an export structure is the liquefaction project. To cut down these costs, investors have a choice of four major commercial structures:
Here, the natural gas producer owns LNG export facilities, the liquefaction units, and the upstream section (exploration and production). He is also responsible for all LNG agreements (both purchase and sale). Revenues come from direct LNG sales. Examples include Qatargas, Sakhalin Island, Tanggu, and Snohvit projects.
This structure separates the owner (producer, buyer, or aggregator) of natural gas from the owner of the export facilities. As a result, the overall project cost is significantly reduced because an independent company provides liquefaction services. However, the gas owner bears all the risks (cost, supply, and demand). Revenues come from tariff payments made by the terminal’s customers. Examples include Damietta, train 4, Freeport LNG and Cove Point facilities.
The liquid natural gas export facility’s owner differs from that of the natural gas producer. The liquefaction project company enters a purchase agreement with the producer. And this is how revenue is generated. This structure allows for more than one natural gas supplier (or producer). Examples include Equatorial Guinea, Angola, Malaysia, and Nigeria.
Here, the investors make their commercial structure by combining some principles from the above three structures. It is common for the host government or independent contractors to demand full participation. They drill the gas, market it, and retain full ownership while the other party benefits from charging a fixed monthly reservation. Examples include Corpus Christi and Cheniere’s Sabine Pass projects.
The commercial structure chosen will impact the project’s overall success. Structures that don’t favour local investors tend to have limited expansion. Remember that the government has the final say on licenses and project approvals. Therefore, factoring them in when choosing a project structure and making critical project decisions is imperative.
Import facilities cost relatively less when compared to the export structures. However, they share the same operating principles (integrated, tolling, and merchant structures). The only difference is that in the absence of an export facility, we constructed a regas terminal. In a nutshell:
Integrated structures – gas owners (who are also the producers) add a regas terminal. Consequently, they can sell this gas to local and distant markets.
Tolling structures – The import terminal charges a fee for everything (regasification, offloading, and storage).
Merchant structures – Import project owner buys LNG, regasifies, and sells to consumers.
The government can participate directly or directly in the national oil company. Its support is vital for investors to get proper approvals. Investors also need support to gain access to the country’s natural gas resources. Other roles include:
Most projects practice a staged project finance structure, while donors finance others directly. LNG projects have more than one financer. Three principal factors influence LNG projects’ financing:
Investors need to be careful with the liabilities of a partnership when sourcing for funding. A “Limited recourse” finance plan takes care of such liabilities. For higher-risk countries, Public-Private Partnerships are the best approach. Other financing considerations include:
The time it takes for an investor’s loan to be approved depends on several factors, but the most important are the size of the project and the amount of risk involved. However, the current unstable gas market is posing a threat to project funding. The potential sources of financing for LNG projects include bank debts, ECAs (Export credit agencies), sponsors, multilateral, equity, and bonds.
LNG prices vary from segment to segment, and setting the right prices is crucial for an investor. Several formulas already exist for setting LNG prices, but most follow oil-based pricing. Europe, however, has recently adopted pricing based on the gas-on-gas method.
In Africa, the goal is to move to LNG imports and establish small-scale LNG projects. As a result, no pricing mechanism has been set yet.
Most African nations are adopting floating liquefaction solutions instead of the usual expensive trains. This is because of the resources’ remote location and environmental issues. Instead of trains, the liquefaction process occurs at sea on the vessel: the same process but with different technology.
In the past, most LNG carriers had an average capacity of 125,000 cubic meters. Today, however, LNG carriers (particularly Qatari ships) have a capacity of up to 266,000 cubic meters, with the largest accommodating 6 billion cubic feet of gas. Modern ships not only provide a more substantial carrying capacity, but they are also more fuel-efficient and have reduced operating costs. The cost of these vessels is equally high. Most cost over $200 million, with a daily charter rate of over $80,000. Ships without secure long-term charters often face economic challenges due to the less lucrative short-term charters available.
In addition, LNG sellers pass on their cost pressures (resulting from gas oversupply) to shippers. This has led to a shift in charter agreements, where shippers are compensated for the loaded leg of the journey but only receive a modest bonus for the return leg. To combat the current downturn in the shipping market, many shippers are converting their LNG carriers into multipurpose floating facilities, with floating liquefaction (FLNG) facilities being the most common. These converted carriers are ideal for African markets to initiate a reliable gas stream for use in power projects. Later, indigenously produced gas can supplement LNG imports. This is after the projects have been undertaken and the domestic market has grown.
Currently, shippers are using smaller marine carriers, known as the “break-bulk” approach. They use even smaller vessels to reload regasified LNG from their regasification terminals. Storage considerations are essential to ensure adequate gas supply during peak demand periods. Finally, for small-scale LNG projects, trucks and small ships are the preferred modes of transportation.
Natural gas production can be categorized into three major stages:
In developing countries, national oil companies dominate the exploration phase with some help from international oil companies. This is due to their lack of experience and the high capital required. Smaller international companies also get involved but fail to complete the projects. Strategic planning determines the overall success of this stage. If this stage succeeds, the rest are more likely to succeed.
The feed gas is sent to a processing plant for further refining. Liquefaction then occurs, which makes LNG more space-efficient for enhanced transportation. However, processing and liquefaction are the most expensive part of the value chain. As such, this stage will benefit more from careful project management and support from local authorities. Proper shipping and domestic market supply arrangements will also hasten capital and cost recovery. They also enhance revenue generation. That will make the investor look “good” to the host country’s citizens.
Specialized LNG carriers transport the liquefied product to a regasification facility. Asians have dominated the shipbuilding industry due to their in-depth design experience. Investors (gas buyers or sellers) then charter these ships for some time (usually the entire project period). However, major project consortiums (like the Qatar projects) build their own ships. The success of this stage depends on the availability of these carriers when needed – usually when the terminal commences operations. The infrastructure, other trucking, and local maritime shipping should also be ready.
An increase in temperature returns LNG to its gaseous form during this stage. The major requirements include a docking facility (for LNG carriers), additional cryogenic tanks (to hold the gas awaiting regasification), and the regasification plant. For this stage to succeed, it will require sufficient regasification capacity.
This is where the host country’s citizens benefit the most. This is due to the establishment of gas infrastructure to facilitate domestic supply. Overall, buyers’ and sellers’ price negotiations and the ability to adapt to any future gas market changes contribute to the success of this stage.
Note
For a successful project, the national government and the contracted international oil companies must coordinate the operations of the entire value chain. Since natural gas is not a commodity business, each party needs to see value addition for the entire project period. For example, the host country will be happy to see cheap local gas supply over the project period, while the drilling oil company will appreciate developed domestic supply infrastructure and enhanced security. It’s a win-win situation.
Short-term contracts are becoming popular due to challenges in long-term project forecasts. LNG projects are generally capital-intensive. The currently projected market oversupply combined with low prices makes it impossible for the entire value chain to appear profitable. However, a dedicated shipping plan might ease the load for long-term projects.
Operational, strategic, and market risks should be shared among the stakeholders (the project developer, host government, upstream developer, EPC contractor, financiers, and LNG buyer). Other risks include:
In African countries, price risk plays the biggest role in LNG investment decisions. The Equator Principles (a risk management framework) used by financing institutions can be applied by gas investors.
Africa is booming with unexplored potential in natural gas and LNG. As the local governments continue to equip their citizens with the relevant skills, investors can now confidently access these resources. It is important to mention that unemployment is a major crisis in most African nations. Therefore, investors must manage high expectations that could lead to social conflicts. Nevertheless, proper planning and forecasting are the most crucial steps for the success of any LNG project. Here is a link to the original report for further reading.
Global maritime transport has traditionally relied on marine and heavy fuel oils (HFO) for propulsion. Whilst these fuels are cost-effective and bunkering is widely available worldwide, they produce harmful emissions. In response, stricter regulations and laws are being implemented to limit ship emissions, leading to the growing adoption of liquefied natural gas (LNG) as a cleaner alternative for marine bunkering fuel.
In light of new regulations aimed at reducing the environmental impact of shipping, fleet owners are looking for alternative solutions. LNG has emerged as a viable, cost-effective alternative, offering significant emissions reductions compared to traditional fuels.
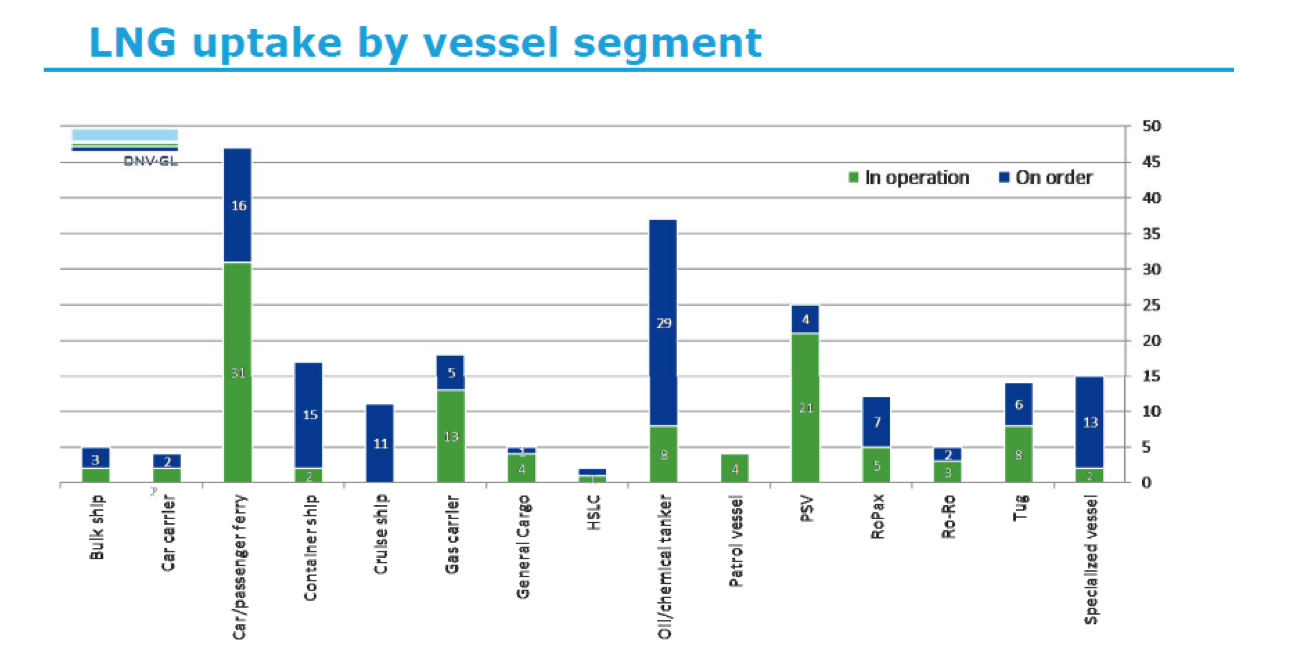
This form of propulsion has been used on LNG carriers for decades (using the boil-off gas from LNG onboard tanks), with about 400-500 in operation today (2017). However, it was only in 2000 that a non-LNG carrier first used LNG as bunkering fuel.
Since then, this market has grown rapidly, with over 100 LNG-fuelled vessels (that are non-LNG carriers) in operation and an equal number on order (2017).
The International Maritime Organisation (IMO) developed the MARPOL Convention (International Convention for the Prevention of Pollution from Ships) to protect the marine environment. The latest annexe, which came into force in May 2005, aims to regulate air pollution emitted by ships, including Nitrogen oxides (NOx), Sulphur Oxides (SOx) and other volatile organic compounds. It also requires the establishment of Emission Control Areas (ECAs), areas in which stricter controls are in place to minimise airborne emissions.
The established ECAs are:
1. Baltic Sea area
2. North Sea area
3. North American area
4. The United States Caribbean Sea area (around Puerto Rico and the United States Virgin Islands)

In October 2016, the IMO established that a global 0.5% (mass by mass) sulphur limit will apply to fuel oil used by ships from 1 January 2020. Within the ECAs, a sulphur limit of 0.10% m/m has been applied since 1 January 2015.
The limits for SOx are:
| Outside an ECA | Inside an ECA | ||
| Prior to 1 January 2012: | 4.50% m/m | Prior to 1 July 2010: | 1.50 % m/m |
| From 1 January 2012: | 3.50% m/m | From 1 July 2010: | 1.00 % m/m |
| From 1 January 2020: | 0.50% m/m | From 1 January 2015: | 0.10 % m/m |
This implies that vessels must either use a compliant fuel or switch from any high sulphur-content fuel to a compliant marine fuel before entering an ECA.
Strict enforcement and inspections will be implemented, especially in European ports. Fines are expected to be levied to offset the economic advantages that may result from disregarding these limits.

Current and Future Sulphur Regulations
LNG primarily consists of methane (CH4), which reduces carbon dioxide (CO2) emissions by up to 30% when used as a fuel. Less nitrogen is present in the combustion process due to the compression rations and methane combustion temperatures, reducing the production of nitrogen oxides (NOx) by up to 85%. Additionally, LNG contains no sulfur, leading to a 100% reduction in sulfur oxide (SOx) emissions. In its liquid form, LNG is non-corrosive, non-toxic, and non-flammable. It is also comparable in cost to traditional marine fuels. However, this depends on the availability of LNG bunkering infrastructure for the vessel, and it also offers the advantage of reduced operating costs.
Increasing stringent regulations will require additional investments to reduce SOx and NOx emissions. Although LNG has a high investment cost, the associated operational savings can be substantial, depending on fuel prices. Alternatives, such as exhaust gas treatment systems, significantly increase the overall cost of the vessel. These systems also require additional space and can increase fuel consumption by 2-3%. Therefore, the use of LNG, which is sulfur-free, presents a viable solution for meeting emission limits.
LNG is widely available, with 20 countries exporting the fuel and 35 importing it. However, existing port infrastructure is focused on these import and export activities, which do not cater to small-scale vessel refuelling. Facilities that provide for the refuelling of ships at ports are termed bunkering and are increasingly being provided worldwide. LNG bunkering infrastructure is currently concentrated heavily in Europe.
LNG is imported in large LNG tankers, with common storage capacities ranging between 80,000 and 260,000 cubic metres. These vessels are substantial in size and cannot be used to deliver LNG to smaller terminals. Instead, smaller LNG feeder vessels, typically ranging from 7,500 to 20,000 cubic meters, supply these smaller terminals in ports. Integrating transfer facilities with existing port infrastructure can be challenging; consequently, bunker vessels are often employed to transfer LNG to ships.
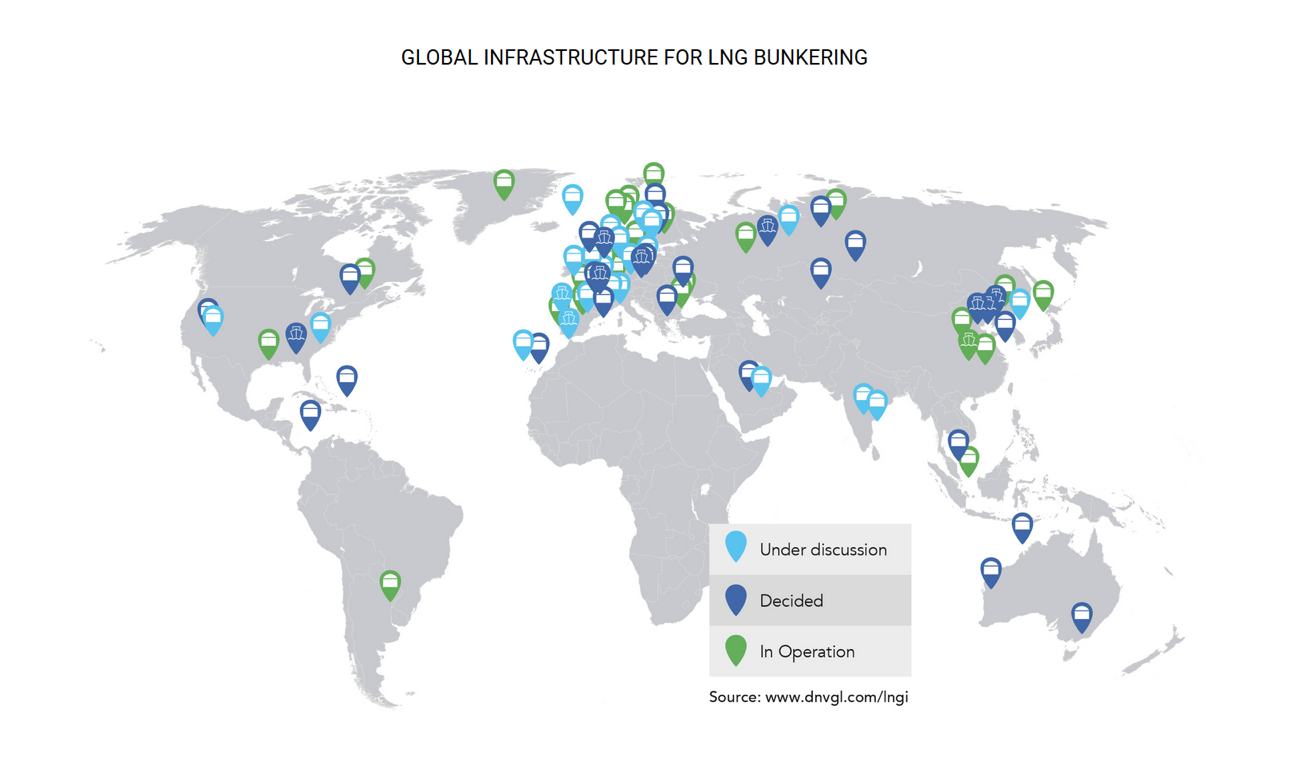
Bunkering requires storage and transfer facilities. LNG can be transferred to vessels from a stationary shore-based tank by pipeline or from mobile units in the form of vessels (ship-to-ship transfer) and trucks (truck-to-ship). The mobile units require their own LNG supply source in the form of strategically placed terminals. The ship-to-ship transfer is the transfer of LNG from a vessel or barge, with LNG as cargo, to another vessel to use as fuel. It can be done at sea or at a port, allowing large volumes to be transferred. The development costs can, however, be high and require space to accommodate the vessels in a port. The truck-to-ship transfer is flexible and costs less, but the quantity of fuel that can be transferred is smaller, and the loading is lower. The ship-to-shore transfer is desirable for large volumes and has a faster turnaround time. However, it requires a large investment to build the facilities in ports. LNG can also be supplied in standardised containers and loaded directly onto the ship.
LNG bunkering is currently available at over 60 locations, with an additional 70 facilities in the planning stages. By the end of 2017, six LNG bunker vessels are expected to be in operation, enabling ship-to-ship bunkering in Northwest Europe and the USA.
The design of LNG storage tanks has to adhere to the various codes. The type, size and tank locations are important considerations. LNG requires approximately four times the storage space required for conventional fuels, so an optimum layout is important. The location of tanks is important from a safety perspective, with their position being restricted by IMO guidelines. The tanks have to be well insulated, and a surrounding safe area is required in case of accidental spillage.
The boil-off gas increases the pressure in the storage tanks. The tanks are, therefore, designed to handle higher pressures and are fitted with pressure relief valves to allow the venting of gas to the atmosphere if the pressures become excessive. Venting gas is only allowed in emergencies and is not a method for pressure control. Venting of the gas is also undesirable from an economic and environmental perspective. The more volatile components of LNG (nitrogen and methane) boil off first, changing the composition and quality of LNG over time. This is known as ageing.
Some methods to manage the boil-off gas are:
The first LNG-fueled vessels that were not LNG carriers used gas-only engines. Dual-fuel (DF) engines can operate on either gas or diesel fuel and can also run on a combination of both, with approximately 70% of energy derived from gas and 30% from diesel. This option is suited to refits of engines that cannot be converted to dual fuel. The most prominent engine manufacturers are currently Wärtsilä, MAN, Caterpillar, HiMSEN, Siemens, Mitsubishi and Rolls Royce.

LNG-ready vessels allow the vessels to be converted to LNG-fuelled vessels in the future. Measures can include structural reinforcements and the correct choice of material to support future LNG tanks, preparations for future gas fuel systems and installation of machinery which can be converted to gas fuel (or is already capable of burning gas fuel). This simplifies a later conversion. An LNG-ready ship is suitable where the current market conditions and/or LNG availability do not make current commercial sense. The additional investment can then be made when the commercial situation is favourable. Being LNG-ready, therefore, increases the flexibility of the vessel, extends its lifetime and can increase its second-hand value.
Converting existing diesel engines for LNG operation is relatively straightforward. This process typically involves changes to the cylinder heads and liners, pistons and rings, connecting rods and turbochargers. Gas rails and admission valves, together with a pilot fuel system and the fitment of storage tanks, were also required.
IMO regulations require a fully redundant fuel supply system. Where gas-only is used, the LNG has to be stored in two or more tanks of approximately equal size. Dual fuel engines are allowed to have a single LNG storage tank, with liquid fuel as a backup.
LNG brings some unique safety considerations when compared with heavy fuel oils. LNG is non-flammable due to a lack of oxygen in the liquid. However, when LNG vapourises, the resulting natural gas becomes flammable when mixed with air in concentrations ranging from 5% to 15% (by volume). Natural gas is both odourless and colourless, and LNG cannot be odorized, making detection without specialized equipment difficult.
LNG’s low temperature (-162 degrees Celsius) can result in severe injuries from direct body contact. It can also render normal ship steel very brittle and fracture it when exposed to LNG.
Due to the large energy content contained in the LNG storage tank, it must be protected. This entails protecting it from possible ship collision and grounding, mechanical impacts, external fires, and BLEVE (boiling liquid expanding vapour explosion).
Despite the associated risks, LNG has proven to be a safe fuel choice for marine transport. The guidelines set by the IMO and the IGF Code ensure that the design and operation of ships adhere to best practices, thereby minimizing potential risks.
The first LNG-fuelled vessel that was not an LNG carrier was the Norwegian ferry Glutra, which came into service in 2000. Within three years, two oil and gas platform supply vessels (PSV) also began operation in Norway. Since then, there has been an increasing rate of new LNG-fueled vessels coming into service. Norway’s tax on NOx, introduced in 2007, and the availability of LNG in the region helped spur the introduction of LNG-fuelled vessels there. Until 2013, all 37 LNG-fuelled vessels (bar one) were based in Norway. The formation of ECA’s in Europe and North America and a steep increase in the price of heavy fuel oils led to more consideration for LNG as a marine fuel. As of May 2017, there are 106 vessels in operation, with an additional 115 on order.

There has also been a diversification in the type of LNG-fuelled vessels, and the areas of operation are expanding.

The gas market has experienced a recent boom, driven partly by the surge in non-traditional (shale) gas production, which utilizes the hydraulic fracturing (fracking) process.
The European Union is actively promoting the use of LNG and funding projects to improve its supply. Current EU policy requires at least one LNG bunkering port in each member state.
The Society for Gas as a Marine Fuel (SGMF) was established in 2014 following a decision by the Society of International Gas Tankers and Terminal Operators (SIGTTO). SGMF is an NGO that aims to promote the safe and responsible operation of LNG-fuelled vessels and develop guidelines for best practices amongst its members. Multi-sector industry organisations, such as SEA/LNG (founded in 2016), are also being formed to create coalitions to promote LNG as a marine fuel. SEA/LNG unites key stakeholders, including shipping companies, LNG suppliers, bunkering companies, ports, and engine manufacturers, to create coalitions that advocate for the use of LNG in marine transport.
EPCM LNG Archive
International Maritime Organisation
International Maritime Organisation – 2020 Global Sulphur Limit
IMO Studies On The Feasibility and Use of LNG as a Fuel for Shipping
IMO – MARPOL International Conventional for The Prevention of Pollution from Ships
SEA\LNG is a multi-sector industry coalition created to accelerate the widespread adoption of LNG as a marine fuel. Our vision is to establish a competitive global LNG value chain for cleaner maritime shipping by 2020.
Natural gas is formed when layers of decomposing plant and animal matter are exposed to intense heat and pressure under the Earth’s surface over millions of years.
This occurrence occurs not only on dry land but also underneath the seafloor. Being sealed off in an oxygen-free environment, the organic material undergoes a thermal breakdown process because the increasing heat and pressure convert the matter into hydrocarbons.
The lightest component of the newly formed hydrocarbon leaves the matter in a gaseous state known as Natural Gas. Once natural gas is completely formed, the odds of the gas being extracted depend on two characteristics of the surrounding rock: porosity and permeability.
Porosity refers to the amount of empty space within a rock’s grains. Sandstone is a typical example of high-porosity rock with large amounts of storage space for fluids such as oil, water and gas.
Permeability, on the other hand, is the measure to which the pore spaces in a rock are interconnected. The higher the permeability of the rock, the easier it will allow fluid to flow through it.

Figure 1. Illustration of Porosity vs. Permeability
This leads to natural gas flowing upwards through rocks with higher permeability due to its low density compared to surrounding rocks.
The natural gas deposits found today are due to upward gas flow through permeable rock until it reaches rock with such low permeability that it can’t flow any further and becomes trapped before reaching the atmosphere.
There are two main categories of natural gas based on origin and location: conventional and unconventional.
Conventional natural gas is often found together with oil reservoir deposits and can be extracted by drilling vertical wells and using traditional pumping techniques. Due to buoyancy, the natural gas will often be found floating on top of the oil or mixed with the oil (Student Energy, Conventional gas).
Unconventional natural gas deposits include shale gas, tight gas sandstone, coalbed methane and methane hydrates, to name a few. Unconventional natural gas is mainly formed deep within the earth, as illustrated in Figure 3. The natural gas deposits trapped deep within these rocks are hard to extract. However, recent technological advances in this field have made it possible to extract a large amount of natural gas from these sources economically. Gas reservoirs are considered unconventional when specialized extraction methods such as hydraulic fracking and horizontal drilling must be used to extract the gas.
Read more about these advances by following the links below:

Figure 2. Illustration of Different Layers of Natural Gas Deposits
Shale gas is trapped deep within the earth in gas-rich shale rock layers. It is extracted using a fracturing or hydraulic fracturing process. Shale gas wells are typically drilled to depths of 1500m – 4000m, with the average wells estimated at 2500m.
Only drilling a vertical well into the shale layer will not release enough gas to make the process economical. This is mainly due to the gas trapped in the low-permeable shale rock. This is why specialized drilling is the only way to extract large amounts of shale gas. Gas-rich shale rock layers make up a large area of the earth’s rock layers, which is exactly why shale gas is one of the largest natural gas resources in the world.
Methane gas deposits are commonly found in underground coal reservoirs, which is considered a natural occurrence. However, methane poses a threat to underground coal mining activities due to the large volumes released and its flammable nature. Therefore, it is feasible to tap into coal seams and extract the gas in a controlled manner, known as coalbed methane, which is a form of natural gas.
Coalbed methane is also referred to as sweet gas, coalbed gas and Coal Mine Methane (CMM). The gas can be extracted using various methods, such as UCG (Underground Coal Gasification), well drilling, and hydraulic fracturing, which are similar to the methods used to extract shale gas. Compared to shale gas deposits, the amount of gas extracted from coal beds is quite small. Coalbed methane has contributed to environmentally friendly extraction methods by injecting carbon dioxide into hard-to-access coal seams, which displaces the trapped methane. This process enhances the recovery of methane-rich natural gas while also storing carbon dioxide underground.
When natural gas flows into rock reservoirs with high porosity but low permeability, it can be referred to as tight gas. Typically, tight gas is held in rocks with pores up to 20,000 times smaller than a human hair, making it nearly impossible for the gas to flow freely.
Because of the nature of the rocks in which it is stored, tight gas commonly requires hydraulic fracturing and horizontal drilling to be released.
Methane hydrates are the most recently discovered and researched form of natural gas. They are formed by methane molecules trapped within a cage of water molecules. Methane hydrates occur naturally in a solid crystalline form, commonly found in sediments in Arctic regions and deep beneath the ocean floor. Although they may resemble ice crystals, methane hydrates will ignite when set alight.
Methane hydrates are estimated to be the planet’s most abundant source of unconventional natural gas (refer to Figure 5). However, there is still much uncertainty about the exact amount of methane hydrate sources. Due to the technical difficulties of extracting this energy source, methane hydrates are considered the most difficult natural gas resource to extract. Economically, extracting the gas remains challenging; only fractions of this resource are found in large enough concentrated volumes to make extraction feasible.

Figure 4. World Map Showing Estimated Methane Hydrate Locations
Certain types of bacteria can produce methane in large quantities through the breakdown of organic matter in an oxygen-free (anaerobic) environment.The bacteria are commonly known as methanogens. With methane being the main component in natural gas, the produced methane-rich gas is considered a type of natural gas referred to as biogenic gas or biogas. Biogenic gas must be differentiated from Thermogenic gas (fossil gas), which is produced from organic material deep in the earth and subjected to high pressures and temperatures. Biogenic gas typically forms closer to the earth’s surface than other unconventional natural gas sources. Thermogenic and biogenic gases have identical properties, but their compositions may differ in some cases.

Figure 5. Typical Composition of Biogenic Gas
Any organic matter is considered a potential source for producing biogenic/bio-gas. Food waste, livestock manure and sewage are just a few examples and can all be broken down into smaller categories. Biogas is considered a major renewable energy source. A popular method for producing biogas is using an Anaerobic Digester system. The gas produced by the digester can then be used to produce electricity from gas-powered generators. It is common practice for farmers around the world to produce biogas from an anaerobic digestion process by using livestock manure or vegetable/food waste as feedstock. Landfill sites can be considered as another major production source of biogas. Large amounts of municipal waste are buried in landfills, which will then start producing biogas, much like an anaerobic digester. The methanogens will start breaking down the organic matter in landfills, such as food waste and newspapers, producing gases such as methane and carbon dioxide. These gases can then be captured and separated before being implemented productively in the energy sector. When implementing biogas, a reduction in greenhouse gas emissions can be expected.
Before implementing any extraction method, the location of natural gas deposits must be established. This can be done using seismic testing, similar to the methods used for locating petroleum deposits. Gas prospectors use seismic trucks or more complex three-dimensional tools to set off a series of small charges close to the earth’s surface, generating seismic waves thousands of meters below the surface in potential underground rock formations such as shale rock and coalbeds. Geophysicists then interpret the results of the seismic waves by using acoustic receivers known as geophones. They then measure the travel times of the waves through the earth, after which a picture is constructed of the subsurface structure and potential natural gas deposits are identified. An exploratory well must be drilled to establish whether the identified gas deposits contain economically viable amounts of natural gas. Once the amount of natural gas is determined, the following extraction methods can be implemented.
Rwanda has implemented the first natural gas extraction method in the world. A natural gas extraction barge is located on Lake Kivu and is the largest extraction plant of its kind in the world. Gas bubbles are extracted from the water and processed accordingly. For further insight into this fascinating development, watch the video and explore the detailed article via the link below:
Hydraulic fracturing, widely referred to as fracking, was developed to extract gas from very low-permeability rock, such as shale rock. The process consists of injecting large volumes of water mixed with sand and various fluid chemicals at high pressure into a well to fracture the above-mentioned rock. This increases the rock’s permeability and the production rate at which the specific well produces gas.
The typical procedure for extracting shale gas through fracking follows these steps:
Once a gas-rich shale rock location is identified, a production well is drilled vertically until it reaches the shale formation. The wellbore then turns horizontally to follow the shale rock. Advances in horizontal drilling have made it possible for a single well to pass through larger volumes of shale, allowing more gas to be extracted.
A steel casing is inserted into the well to ensure it remains open and to protect the wellbore’s integrity. Cement is then pumped into the well and forced up the outside of the casing to seal the well, preventing fracking fluid, natural gas, chemicals, and produced water from leaking into surrounding groundwater supplies.
Once the well is sealed, small explosives are detonated in the horizontal section to create holes at specified intervals where fracking will occur. Fracturing fluid is pumped into the well at controlled pressures to fracture the rock several hundred meters from the well. Sand mixed with the fluid holds the cracks open when pumped out. After fracturing, gas flows freely to the surface, where it is collected in a controlled manner.
Underground coal gasification is an industry process that converts coal into gas while it is still underground. It involves drilling wells into the coalbed layer. Oxygen and water are then injected into the well. Once the well is injected with the appropriate amounts of oxygen and water, the coal is partially buried underground. The burned coal then produces a type of natural gas that starts flowing towards the surface. An animation of the USG process can be seen in the video below:
Vertical well drilling is a common method for extracting natural gas. After exploration, well locations are determined. Many natural gas reservoirs, particularly conventional ones, are found at relatively shallow depths. Horizontal drilling or hydraulic fracturing is not necessary to extract natural gas from these reservoirs because the gas pockets are typically found close to the earth’s surface and not in tight rock layers. These natural gas deposits are primarily of bacterial origin, continuously producing gas at relatively shallow depths, and are classified as biogenic gas. Vertical wells are drilled at depths varying from 300m-800m until a gas pocket is reached and gas starts flowing toward the surface. Horizontal drilling may be considered in some cases to interconnect wells and gas pockets in an underground system to increase the flow of wells. These natural gas wells usually produce gas at low pressures varying from 0.2 to 4 bar.
Continuous Carbon Capture Process: In recent years, greenhouse gases have been identified as the major culprits of climate change, and carbon dioxide constitutes as much as 70% of the greenhouse gases emitted (U.S. Department of Energy, 2007). As a result, carbon taxes are increasingly being implemented worldwide to curb carbon dioxide emissions. Technologies that can capture carbon dioxide effectively and with a low parasitic load on the system will enable any operator emitting carbon dioxide to generate financial savings while contributing to the preservation of our environment.
Many processes exist on an industrial scale that capture carbon dioxide, but all of these processes are extremely energy-intensive. The main reason these processes are so energy-intensive is due to the batch or cyclic nature of the processes. Changing the temperature or pressure in the carbon dioxide capture system requires large amounts of energy to drive these processes. A continuous carbon dioxide capture process will, per definition, be more energy efficient.
This article documents the current hypothesis for developing and testing a continuous carbon dioxide capture system using Stirling Coolers as the driving force.
A Stirling cycle cooler is a member of a family of closed-cycle regenerative thermal machines, including heat pumps and refrigerators, known collectively as Stirling cycle machines. In any refrigeration cycle, including the reversed Stirling cycle, work input is required according to the second law of thermodynamics. This is achieved by shuttling the gas in the system backwards and forwards between the hot end and cold end spaces so that the system’s temperature during compression is, on average, higher than during expansion. As a result, the work done on the gas during compression is greater than the work done by the gas during expansion, as illustrated in Figure 1. Accordingly, the hot and cold end gas spaces are also referred to as the compression and expansion spaces, respectively. Furthermore, for operation as a refrigerator, heat must be rejected via a heat exchanger at the hot end, and heat must be absorbed from the space to be cooled via a heat exchanger at the cold end.
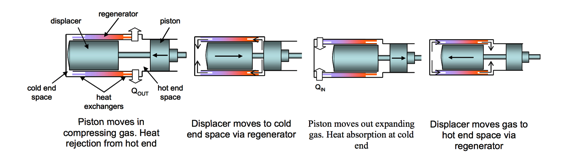
Figure 1. Piston and Sisplacer Movements During a Stirling Refrigeration Cycle.
Stirling engines have the advantage of being able to effectively convert heat energy as a driving force into mechanical work. Stirling coolers reverse the cycle, through supplying a mechanical driving force, heat energy is generated and removed by a heat exchanger, thus the cold end of the cylinder incrementally reduces its temperature to reach very low temperatures for relatively little work done. The figure below (source: Ray Radebaugh (NIST) 1999) illustrates Stirling cryocooler efficiency at 80K compared to traditional refrigerant, which is driven by cryogenic cooling systems.

Figure 2. Cryocooler Performance Comparison
Cryogenic Carbon Capture processes have proven to work well, regularly recovering over 95% of the CO2 content in the gas stream. Due to the relatively high sublimation temperature of CO2, this is basically a single step. Typically, the condensation or sublimation temperatures for effluent or hydrocarbon gasses are much lower, enabling a very successful separation of the CO2.
As seen in the phase diagram below, for gas pressures below 5.1 1 atm, no liquid phase for CO2 exists, and thus, it sublimates directly from gas to solid state. This enables cryogenic processes to directly freeze out the CO2 from a gas stream below 5.11 atm.

Figure 3. Carbon Dioxide Phase Diagram.
Freezing out the CO2 directly from a gas phase requires a surface for the crystals to propagate on, which is the limiting factor in the existing cryogenic carbon capture processes. This limitation gives them batch characteristics and requires energy state changes for operation. To make a continuous cryogenic carbon capture process more energy-efficient and feasible for industrial use, an infinite surface area for CO2(s) propagation would be required. Traditional heat exchanger design clearly doesn’t allow an infinite surface area, which would, in any case, require an infinite amount of energy to drive the sublimation of the CO2.
Since our aim is a continuous process that doesn’t require cyclic changes in temperature or pressure to remove the CO2(s) from the gas stream, we clearly need to think creatively about heat exchanger designs.
To ensure the heat exchanger conforms to the process requirements, we must define the processes for removing the CO2. Our organisation’s current need is to enrich a bio-gas feed stream from an anaerobic digester (removal of CO2 and water vapour). The typical composition of the bio-gas contains more than 38% CO2 and varying amounts of H2O vapour, with the main constituent being methane (CH4), less than 4% nitrogen (N2) and very low concentrations of hydrogen sulfide (H2S). Since the primary objective is the removal of CO2 and the removal of water vapour is well established, we will only focus on CO2 removal as a process constraint. This process will typically be included after the H2O vapour has been expelled from the system.
Since it has already been established that a conventional heat exchanger design will not meet our requirements, we must utilize a different approach for the required heat exchange. The author’s approach was to conceptualize a mass transfer process rather than focusing solely on energy (heat) transfer. This process also exchanges energy and provides an opportunity to separate different components based on their phase change properties.
The first process that comes to mind is a traditional scrubber column, which contacts liquid and gas streams to separate components from one stream, with the components being absorbed by the other. This process also facilitates energy exchange between the streams. If the selected liquid stream does not dissolve any components of the gas stream, nor is dissolved into the gas stream, only energy transfer will occur, functioning as a “Direct Contact Heat Exchanger.”
The liquids stream, cooled down sufficiently low, and if sufficiently dispersed (while ensuring sufficient mass to ensure heat exchange from the liquid to the gas can still occur), will increase the surface volume of the “heat exchanger” substantially, supplying a very large area for the propagation of the CO2 crystals. Consequently, CO2 crystals form on the surface of the heat exchanger liquid droplets. If the liquid stream and solid CO2 are separated, the liquid can be cooled again and recycled to the scrubber column, creating an infinite surface area for CO2 crystal propagation and enabling true continuous cryogenic carbon capture.
Since the preliminary idea for a Direct Contact Heat Exchanger (DCHE) with a theoretical infinite surface area has been established, we must conceptualize the supporting equipment to enable its successful operation.
As discussed in the above section, the preliminary equipment will be based around an absorption or scrubber column. Typically, these columns come in various arrangements depending on the intended separation of components from the different feedstocks. For the DCHE arrangement, a counter-current scrubbing column is recommended to optimize the temperature difference (DT) between the gas stream and the heat exchange liquid (similar to the concentration difference in a mass transfer process).
Since the propagation of the CO2 crystals occurs on any possible surface and can consequently create system blockages, the internal design of the scrubbing column should not include any trays and should only be composed of a single spray head (possibly multiple nozzles to maximize the liquid dispersion).

Figure 4. Proposed Scrubber Column (single head layout).
The next step would be to conceptualize the heat-exchange liquid. This approach is very straightforward. As mentioned in the preliminary constraints, the heat exchange fluid should not allow any mass transfer from the gas stream, including the CO2, to it, or allow any of its mass to be transferred to the gas stream or the CO2 stream. Further, since we will be operating the column below the sublimation temperature of the CO2, the heat exchange liquid should have a lower freezing point while remaining sufficiently viscous at the operating temperature to allow for easy pumping and dispersion.
A detailed investigation is needed to identify a suitable heat exchange liquid. Hydrocarbon ring compounds containing fluorine (such as polytetrafluoroethylene) or chlorine atoms immediately come to mind.
It is reasonable to expect that the CO2 crystals forming on the surface of the cold heat exchange liquid will create a slurry when collected. The next step is to conceptualize the separation process for the liquids and solids.
Yet again, it is easier to turn to existing technology and possibly change it slightly to fit our process requirements. A 2-phase decanter centrifuge employs a rotational moment to separate components of different densities, making it ideal for slurry separation.
In typical horizontal decanter centrifuges, the rotating assembly is mounted horizontally with bearings on each end of a rigid frame, which provides a good sealing surface. The typical arrangement must be adapted to separate the CO2 crystals from the heat exchange liquid. Instead of the typical horizontal arrangement with the feed being fed in the middle of the centrifuge, the proposal is to orientate the decanter centrifuge at 45°, with the scroll and housing submerged under the liquid level, while employing a preliminary porous scroll housing to allow the heat exchange liquid to drain. The scroll discharge screw then forces the solid CO2 crystals to one end of the bowl to be discharged as solid CO2 ice. This arrangement will allow the centrifuge to function as a scroll feeder with a higher-than-normal rotational force and possibly compress all the liquid out of the solid CO2 by changing the scroll vane geometry.
As discussed in Section 2 above, the heat exchanger liquid’s cooling source will be a Stirling engine.
The conceptual idea for the “direct contact heat exchanger” and its supporting equipment has been established and needs to be defined for feasibility development. This design process set point is required for evaluating the preliminary concept and will assist in developing it into a feasible proposal. From this feasible proposal, a working pilot facility can be constructed to prove the concept and develop it into an acceptable process for industrial applications where needed.
The process layout to be tested for feasibility is illustrated below:

Figure 5: Conceptual Layout of Envisioned Continuous Carbon Capture Process.
The above layout, along with the descriptions in the previous sections, will serve as the conceptualization set point from which further feasibility studies will proceed. All components will be theoretically assessed for their ability to deliver on their intended requirements individually, and then the process will be assessed as a unit.
EPCM Consultants have proposed the above concept to the University of Pretoria’s engineering department in South Africa to assist with its feasibility development. A team selected by the university will carry out further development to create a feasible proposal and, ultimately, a working prototype. All progress will be included in future revisions of this article.
In this article, EPCM reviews the Process of Helium Recovery from Natural Gas and conventional processes for recovering helium from natural gas. It examines the potential of emerging technologies for more efficient helium production processes, with the main focus on Southern Africa’s new helium-rich gas reserves.
Helium is a valuable noble gas with unique properties used in various applications, including the medical, nuclear and space industries (Soleimany, Hosseini & Gallucci, 2017). Despite its status as the second most abundant element in the universe, the only commercially viable source for helium recovery is natural gas reserves (Sunarso et al., 2016). Helium extraction from gas sources containing more than 0.05 % (vol) helium has been proven economical (Kim, 2014).
Separating helium from natural gas aims to increase natural gas’s heating values and helium recovery for higher returns (Mehrpooya & Shafaei, 2016). In 2010, the global demand for helium reached 30,000 tons, amounting to US$1 billion (Soleimany et al., 2017). The demand in the next two decades is expected to double (5-7 % increase per annum) (Sunarso et al., 2016). Helium extraction facilities currently in operation are listed in Table 1.
As can be seen, the United States accounts for nearly 71% of global helium production plants, followed by Algeria, Qatar, Poland and Russia. Currently, 21 nuclear power plants (mostly in Asia) with 150 reactors are under construction, which will also require enormous amounts of helium for cooling systems in the near future (Rufford et al., 2014). It is clear that current production plant capacities fail to meet the projected demands, leading to constant yearly increases in helium prices (illustrated in Figure 1).
Recently, two valuable helium-rich natural gas resources have been discovered in Southern Africa. South Africa’s Virginia gas field contains up to 4 % helium, and Tanzania’s Craton basin in Rukwa province shows samples that contain up to 10 % helium. Table 2 provides a range of compositions of helium-rich gas from various global natural gas fields.
Table 1: List of Global Helium Extraction Plants (West, 2009)

Figure 1: Helium price comparison with other natural resources (USGS, World Bank)
Table 2: Composition of helium-rich natural gas fields (Häussinger et al., 2005)

Indicated in the high helium compositions recently discovered in Southern African gas reserves compared with other helium “rich” resources. This highlights Africa’s untapped potential, waiting to be unlocked, even before considering neighbouring countries’ rapidly growing natural gas discoveries.
This article reviews conventional processes for recovering helium from natural gas. It examines the potential of emerging technologies for more efficient helium production processes, with a main focus on Southern Africa’s new helium-rich gas reserves.
When performing helium extraction from natural gas, the helium is first separated/recovered from the bulk fluid, where further purification is done. Figure 2 is a block-flow illustration of a general process for helium recovery from natural gas (Soleimany et al., 2017).

Figure 2: Schematic representation of a typical helium recovery process from natural gas (Soleimany et al., 2017)
Natural gas compositions vary depending on resource locations. Common natural gas resources consist of 30-90 % methane and light hydrocarbons. In addition, other gases that form along with natural gas are nitrogen, hydrogen sulphide, water, carbon dioxide, and traces of heavy metals (Hosseini, 2009; Hosseini & Najari, 2016).
Tanzania’s Itumbula, Rukwa Spring’s deep crustal gas compositions illustrated 8-10.2 % helium with approximately 90 % nitrogen (Helium One, 2017). The Virginia microbial gas field in South Africa has a proven 4 % and probable 10 % helium-containing reserve, with the remaining gases being methane and mostly small amounts of water vapours.
To extract helium, all other impurities and contaminants must be removed following industry standards. The process of extracting and producing liquefied helium from natural gas consists of six steps (Soleimany et al., 2017):
The pretreatment process is imperative for removing acid gases, water and heavy metals (commonly mercury) before entering the refrigeration and liquefaction process. Helium has an extremely low boiling point (seen in Table 3). Therefore, any helium in the natural gas feed to the LNG production plant is concentrated in the overhead product of a nitrogen rejection unit (NRU). This process is conventionally a cryogenic distillation process where the helium recovery is integrated with the NRU. It is desirable to have a helium recovery unit; otherwise, the remaining helium is vented to the atmosphere along with nitrogen (Rufford et al., 2014).
Table 3: Physical properties of helium and other gas components encountered in helium recovery (Rufford et al., 2014)

Gas separation processes are divided into three categories: cryogenic processes, pressure swing adsorption (PSA) and membrane separation (Bakhsh et al., 2007; Crawford, Coyle & Anantharaman, 2010).
In cryogenic technologies, separation is achieved at temperatures below -65 ºC. Cryogenic separations can accomplish up to 90% helium recovery. Cryogenic processes are divided into two groups: multi-flash cycles and high-pressure distillation column processes (Victory, Miles & Oelfke, 2009).
PSA methods are based on the adsorption of gases on solid surfaces and operate at near-ambient temperatures (Rufford et al., 2014). These processes are mainly used in the pretreatment, nitrogen rejection, upgrading and purification steps of helium recovery from natural gas (to be discussed).
Membrane technologies effectively separate gas mixtures using synthetic membranes made from various materials based on the theory of Fick’s Law. They have not yet advanced, like cryogenics and PSA separation methods. However, extensive research is currently being undertaken due to the potential economic incentive membranes may have over cryogenic distillation and PSA processes (Sunarso et al., 2016).
Cryogenics are employed in LNG production plants, where helium is extracted from the NRU after the feed gas is liquefied. While cryogenic methods for LNG production are extensively covered in other literature, this discussion will focus on the key design features of NRUs utilizing cryogenic techniques.
The four fundamental cryogenic processes used in NRUs are multi-stage flash separators, single-column heat-pumped processes, double-column processes, and dual-column cycles (Agrawal et al., 2003). The choice of process depends on factors such as the feed gas flow rate, its composition, and the helium concentration, all of which must align to ensure economically viable helium recovery. Multi-flash separator processes have higher energy requirements compared to cryogenic distillation column processes but have lower capital costs and production overhead vapour streams with low helium concentrations. Depending on the feed gas composition, a single-stage flash separator process produces nitrogen-rich overhead vapours containing 1-3 % helium. 50-70 % helium can be achieved from more complex, double-column NRU processes when operating in partial condensation mode, where nitrogen and small amounts of methane, hydrogen, neon, argon and carbon dioxides are also present (Agrawal et al., 2003; West, 2009).
Figure 3 illustrates a multi-stage flash process. The dissolved nitrogen and helium are removed from the LNG feed by reducing the pressure over a series of flash vessels.
In each flash stage, the helium is vapourised with the nitrogen, and liquid natural gas is used to pre-heat the feed. This process can also be applied to the overhead product of the NRU after it is cooled (partially condensed) to separate the gases (West, 2009). Crude helium concentrations in the products depend on the feed composition, pressure drops and temperature changes.

Figure 3: Schematic of a multi-flash process for recovering helium from natural gas (Rufford et al., 2014).
In the single-column heat-pumped process (Figure 4), a purified NG feed is pre-cooled in the main cryogenic heat exchanger (MCHE; against the rejected nitrogen) and then fed to a high-pressure column (13 – 28 bar). The vapour ejected from the top of the column contains nitrogen and helium. A closed-loop (methane used as working fluid) supplies heat to the reboiler and cooling duty to the condenser. The methane is condensed and drawn from the bottom of the tower, whereafter it is flashed over a control valve (reduction in pressure). The feed gas and rejected nitrogen from the top of the column are used to heat the methane before being compressed into downstream facilities. This process produces a nitrogen-rich stream containing approximately 1-3 % helium, depending on feed gas conditions (Rufford et al., 2014).

Figure 4: Single-column heat-pumped process (Agrawal et al., 2003)
Häussinger et al. (2005) describe a simplified process flow scheme of a modern, double-column process for nitrogen rejection and helium recovery from natural gas, as seen in Figure 5.
The feed gas is cooled against cold product streams in a cryogenic heat exchanger (main feed/product HX) and is fed to the bottom of the high-pressure column. In this high-pressure column, the helium is recovered from the feed at typical operating pressures between 10 – 25 bar. Reflux for the low-pressure and the high-pressure column is provided by the column’s partial condenser (not illustrated). The helium is contained in the non-condensed part of the high-pressure column’s overhead vapour, which is then directed to subsequent stages for further processing and purification.

Figure 5: Double-column process for nitrogen rejection and helium recovery (Häussinger et al., 2005)
The final separation of nitrogen from methane at the bottoms of the high-pressure column is carried out in the low-pressure column. The nitrogen-rich overhead product of the low-pressure column is heated in a heat exchanger against the bottoms of the high-pressure column. A reboiler is required at the bottom of the low-pressure column to achieve a low nitrogen concentration in the methane-rich residual product. The reboiler duty is provided by condensing the top nitrogen stream from the high-pressure column. The required heat transfer is possible because of the higher nitrogen stream temperature relative to the methane temperature from the bottom of the low-pressure column. A methane-rich stream is produced at the bottom of the low-pressure column, pumped to elevated pressure, vapourised, and heated against the natural gas feed (Rufford et al., 2014).
Modern cryogenic helium recovery processes are significantly more complicated than the simplified single-column and double-column systems described. The dual-column cycle shares many common features with the double-column process but has a higher level of integration between process and refrigeration streams. Depending on the local feed gas composition and available product markets, the complete, integrated cryogenic process may include the recovery of heavier hydrocarbons, fuel gas and nitrogen fractions, in addition to the recovery of crude helium (Rufford et al., 2014).
The crude helium from the NRU must be upgraded to a helium concentration of at least 90 % before liquefaction. The impurities still contained in the crude helium, like nitrogen, methane, hydrogen and sometimes neon, must be removed in several stages. The upgrading process includes condensation of the bulk components, catalytic oxidation of any trace hydrogen, separation of water, carbon dioxide and oxygen from the reactor by condensation of the water and then within a PSA unit, and removal of the final traces of nitrogen in another PSA unit. The product from these steps can approach helium purities of 99.995 % (Daly, 2005).
Figure 6 is a schematic representation of a typical process to purify upgraded helium. The upgraded helium is mixed with air (provision of combustion oxygen), heated to above 300K and compressed through a catalyst bed to oxidise traces of hydrogen or remaining hydrocarbons. The product from the reactor is cooled to condense any water formed and sent through a water separator, from where the top gases are fed to a PSA unit. Molecular sieves in the PSA unit may be used for further dehydration, carbon dioxide and oxygen capture (Rufford et al., 2014).

Figure 6: Schematic of the upgraded helium purification process (Agrawal et al., 2003)
To attain a helium purity of 99.995 %, the hydrogen-free gas needs to flow through a low-temperature PSA unit (Lindemann et al., 2010) and/or additional cryogenic condensation processes to remove nitrogen to less than 10ppmv (Agrawal et al., 2003). Four-bed PSA molecular sieve units containing an adsorbent such as zeolite 4A are commonly used. The nitrogen blowdown gas from the PSA purification unit is compressed, dried and recycled to the inlet of the upgrader, where it is combined with the crude helium feed (Rufford et al., 2014).
Common industry processes for liquefying helium are based on isenthalpic throttling of the purified helium across a Joule-Thomson valve. The purified helium is compressed (to 20 bar) and pre-cooled to 80K with exhaust from the helium expander or liquid nitrogen, then cooled to 20K with hydrogen refrigerant or below 80K again with exhaust from the helium expander (Agrawal et al., 2003; Häussinger et al., 2005). The free expansion of the compressed gas produces the final liquid helium.
A case study is presented by (Lindemann et al., 2010) on a recently commissioned (2010) helium production facility in Australia based on direct helium separation from natural gas using cryogenic separation. The helium plant at the Darwin LNG production facility comprises a 3 % crude helium feed from an NRU (downstream of the main cryogenic heat exchangers). The plant can produce 2.6 tons per day of liquid helium (860 litres per hour) with a purity of 99.999 %. This process utilises a two-stage cryogenic flash process, a hydrogen oxidation reactor and two PSA units.
The nitrogen-rich feed is compressed to 2 bar and upgraded by cooling to 80.5 K in the first nitrogen removal stage, where part of the nitrogen is condensed to give a helium-enriched stream of 26 %. The gas is then warmed to ambient temperature, compressed to 31 bar and mixed with air to feed the hydrogen oxidation reactor. The water and carbon dioxide formed are removed in a PSA unit. After hydrogen removal, the helium-enriched gas is cooled to 81 K (second nitrogen condensation stage), producing a 93 % helium vapour stream. The upgraded helium stream is further cooled to 68 K and then flashed to provide a product of 99 % helium. Traces of nitrogen are removed (< 5 ppmv) in the final purification stage using a cryogenic PSA unit.
The world’s largest helium production facility (Ras Laffan Helium Plant in Qatar), with a production capacity of 17.7 tons per day and feed gas composition of 0.04 % helium, is based on the same cryogenic distillation principles. Each of the seven LNG production trains has its own helium recovery unit installed at the cold end of the LNG process to upgrade the crude helium produced (Daly, 2005).
Adsorption-based processes are mainly used during the pre-treatment process to remove water, carbon dioxide, hydrogen sulfide and other impurities from the feed natural gas. This process is also used to remove trace impurities of nitrogen and methane during helium purification (Tagliabue et al., 2009). Gas separation using adsorption comprises two steps: adsorption and desorption.
During adsorption, a porous solid bed selectively adsorb the higher affinity gas to the adsorbent bed and produces a gas stream enriched with the less strongly adsorbed gas component. Once the solid is saturated, the adsorbent must be regenerated. In the desorption column, the gaseous product is enriched with the strongly adsorbed component (Rufford et al., 2014).
The technologies that deploy this method are temperature-swing adsorption (TSA), PSA, fluidised adsorbent beds and moving adsorbent beds. TSA methods regenerate the gas using external heat to increase the temperature in the desorber, whereas during PSA methods, regeneration is conducted under low temperatures but by releasing pressure and purging the bed (Tagliabue et al., 2009). Fluidised and moving bed operations are less common for industrial gas separations than the cyclic-batch fixed bed operations of TSA and PSA (Saeder & Henley, 2006).
From Table 3 it is seen that the molecular size of helium (2.60 Å) and hydrogen (2.89 Å) are similar with both having very low boiling temperatures. This indicates that PSA processes used for hydrogen purification potentially have many features similar to helium purification processes. Rufford et al. (2014) stipulate the potential of new technologies under development for hydrogen purification that can also apply to helium purification. In general, modern PSA units for hydrogen purification use layered adsorbent beds with three to four adsorbents (silica, alumina for water, activated carbon for carbon dioxide, and 5A zeolite for methane and nitrogen; Ritter & Ebner, 2007). Similar multi-layer bed designs are researched for Helium recovery processes (Baksh, 2010).
It is important to note that PSA units are designed to treat a feed stream that is already upgraded (helium > 90 %); otherwise, the adsorbent will be too readily saturated by non-helium components. PSA is only used during upgrading and purification stages where nitrogen and traces of methane are removed from the helium (downstream of NRUs) (Daly, 2005; Lindemann et al., 2010).
Commercially available zeolites and narrow pore-activated carbons with reasonable nitrogen adsorption capacities make suitable adsorbents for helium purification. Table 4 lists commercial adsorbents used in industrial hydrogen purification processes that can potentially be used in PSA processes to separate heavier components from helium. Baksh (2010) also patented the use of calcium and lithium-exchanged 13X adsorbents.

Table 4: Commercial adsorbents with potential for PSA units for helium upgrading and purification (Rufford et al., 2014)
Rufford et al. (2014) discuss two case studies of direct helium recovery from natural gas using adsorption-based processes. The first is India’s Oil and Natural Gas Corporation PSA Pilot Plant, with a production capacity of 23 kilograms per day, having a feed gas containing 0.06 % helium (88.5 % methane, 9.86 % heavier hydrocarbons, 1.18 % nitrogen and 0.4 % carbon dioxide). This process has four stages: (1) pretreatment of the feed gas using PSA, (2) recovery of methane gas onto an AC adsorption bed, (3) upgrade of helium from nitrogen on a zeolite 13X adsorption bed, and (4) helium purification also using zeolite 13X adsorption bed. Although this plant illustrates the possibility of using PSA methods throughout the process, the plant only recovers 65 % of the helium in the feed gas. Conventional cryogenic processes can easily recover more than 95 % helium from the feed gas.
The second case study discussed is a US patent (no. 5542966) where a natural feed gas containing 4 % helium, 26 % hydrocarbons and 70 % nitrogen flows through a two-stage activated carbon PSA process. The production capacity is 550 kilograms per day, and a recovery of 95 % helium is reported. Although the helium recovery of 95 % is approaching cryogenic distillation process results, the nitrogen in the feed gas is high compared with conventional natural gas fields (< 10 %, 70-90 % methane). To apply to Southern Africa’s gas reserves, this process must be tested with a higher methane composition feed gas (> 80 % hydrocarbons).
Gas separations performed through membrane methods are the permeation of gases through a homogeneous membrane. This is a solution-diffusion phenomenon where the ability of a membrane to separate components of a gas mixture depends on the selectivity or separation factor of gaseous components. This, in turn, is a function of the gas solubility and diffusivity coefficients (Häussinger et al., 2005). Because of the small molecular diameter of helium compared with other natural gas components, its diffusivity and, therefore, permeability in most membranes are greater, allowing for the separation of helium to be possible.
A range of membrane processes has been designed in patents, and research has been developed to recover helium from natural gas for the past 40 years (Stern et al., 1965; Scholes & Ghosh, 2017). For recovering helium directly from natural gas, membranes have demonstrated that this separation is possible when combined as two or three stages in series with recycle streams (Figure 7).
Multi-stage systems exhibit large pressure drops of the helium-rich permeate across membrane units, requiring inter-stage compressors. The recompression between membrane stages increases membrane systems’ capital and operating costs. However, these designs can enable natural gas containing as low as 1 % helium to be purified to a very high concentration while utilising existing membranes with high helium/methane selectivities (Scholes & Ghosh, 2017).
No data on the performance of helium recovery plants from natural gas utilising membrane technology has been published in the open literature, though there are various processes in patented literature (Rufford et al., 2014; Scholes & Ghosh, 2017).

Figure 7: Two- and three-stage membrane process illustrations (Scholes & Ghosh, 2017)
Two- and three-stage membrane stages in series, with recycles, have been reported as feasible for recovering and purifying helium from the overhead gas of NRUs (Scholes & Ghosh, 2016). This is due to the high nitrogen concentration and reasonable compression ratios for helium/nitrogen selectivities above 20.
Alders, Winterhalder & Wessling (2017) conducted a techno-economic comparison between various membrane-based processes for helium recovery and enrichment. Two scenarios were considered: (1) helium and nitrogen were recovered after cryogenic distillation of the natural gas, and (2) direct separation of helium from natural gas. Scenario (1) investigated the separation of nitrogen and helium by comparing (i) a pressure relief distillation, (ii) a two-stage membrane process and (iii) a second low-temperature distillation. Scenario (2) investigated direct helium removal from natural gas by comparing (i) multi-stage pressure relief, (ii) a two-stage membrane process and (iii) a three-step membrane process.
Figure 8 (pressure-relief distillation after cryogenic distillation), Figure 9 (two-stage membrane process after cryogenic distillation) and Figure 10 (two-stage distillation system) schematically illustrate the processes for Scenario (1).

Figure 8: Combined distillation and pressure relief process (Alders et al., 2017)

Figure 9: Hybrid process integrating distillation and membrane gas separation (Alders et al., 2017)

Figure 10: Two-stage low-temperature distillation system (Alders et al., 2017)
The evaluation confirmed that the hybrid process integrating the distillation and membrane technologies resulted in the lowest treatment costs, was most favourable concerning operating costs for short amortisation periods, and showed the highest helium recovery (94.2 %). All three processes were based on the same natural gas feed conditions (500 kmol/h, 80 % methane,19 % nitrogen and 1 % helium).
Figures 11 (multi-stage pressure relief), 12 (two-stage pressure relief) and 13 (three-step membrane process) show processes investigated for Scenario (2).

Figure 11: Multi-stage pressure relief system (Alders et al., 2017)

Figure 12: Two-stage membrane process (Alders et al., 2017)

Figure 13: Three-stage membrane process (Alders et al., 2017)
The results illustrated that the three-stage membrane process required the lowest treatment and cost and remained the most favourable option for all investigated prices while still achieving a helium recovery of 90.2 %. Scholes, Ghosh, and Ho (2017) state that membrane materials with even higher selectivities than polypyrrole, used in this investigation, do not further reduce treatment costs.
Extensive research has been undertaken to investigate the most favourable membrane materials for helium recovery from natural gas. The first helium extraction membranes included tubular silicate, quartz glass membranes, and polymeric membranes. Recently reported membranes include those constructed from ultra-microporous silica (Barboiu et al., 2006), molecular sieve carbons, porous graphene (Schrier, 2010), titanium silicates (Li et al., 2011), polyamides and mixed matrix membranes of polyimides and zeolitic imidazolate frameworks (Bernado, Drioli & Golemme, 2009).
Sunarso et al. (2016) surveyed five different membrane materials regarding their hydrogen and helium permeation performance and the related stabilities during permeation processes. Membranes evaluated included: silica, polymers, zeolite, metal-organic framework and mixed matrix membranes. The results illustrated that each membrane can be used for recovering helium, but it contains advantages and disadvantages in terms of permeability, selectivity, stability, cost, synthesis procedures, and reproducibility.
The literature on membrane materials performance shows that ultra-microporous inorganic membranes and glassy polymeric membranes are the most promising membranes for achieving successful helium recovery through membrane-based processes. Various polymeric membranes have been implemented successfully to upgrade crude helium to 90 % purity, which has energy duties comparable with other crude helium separation technologies (Scholes & Ghosh, 2017).
Table 5:Advantages and limitations of helium recovery technologies

The future success of membrane-based helium production from natural gas process technologies will depend on developing high-performance membranes and designing proper membrane separation processes for industrial applications. The focus must be on implementing patented low-cost modules for natural gas reserves to evaluate their operability and distinguish between the most optimal designs.
There are considerable opportunities to improve the efficiency of PSA processes for helium recovery through improved designs: optimised processing conditions by operating at, for example, cryogenic temperatures; and improvements in adsorbent materials (Rufford et al., 2014). A challenging approach is to develop helium-selective adsorbent materials, which can possibly reduce the size and energy requirements of PSA beds. Currently, no commercial adsorbents with sufficient helium capacity and selectivity are available to realise such an industrial application (Rufford et al., 2014).
There remains scope for developing and optimising various helium recovery process layouts featuring cryogenic distillation, PSA modules and membrane separation (Rufford et al., 2014). Although detailed technical feasibility on energy requirements and process economics of these integrated system designs still need to be conducted, it would appear that integration of the processes is key to providing the most economical solution for producing helium from natural gas. Such a process may include cryogenic distillation to recover helium from natural gas, coarse separation of crude helium using membrane technology, and final PSA purification.
Concerns regarding inequality between helium demand and production can be addressed by developing other improved helium recovery processes than the conventionally high capital-intensive cryogenic separation technologies.
To economically recover lower content helium (< 0.05 %) from natural gas and distinguish between optimal designs for Southern Africa’s helium-rich gas reserves, an integrated approach between cryogenic distillation for direct recovery of helium from natural and membrane-/adsorption-based processes for crude helium upgrading and purification must be designed.
Adsorption-based processes, excluding cryogenics, are not recommended for direct helium recovery from natural gas. Limitations were found for separating impurities and other gases from helium in low-quality gas streams, resulting in lower helium recoveries.
Although membrane-based technologies cannot yet compete with cryogenic separation for direct helium recovery from natural gas, there will be continual future interest and research developments due to the potential energy cost savings and lower associated process costs.
Agrawal, R, Herron, DM, Rowles, HC and Kinard, GE (2003), “Cryogenic Technology”, Kirk-Othmer Encyclopedia of Chemical Technology, Volume 8, John Wiley & Sons, Hoboken, NJ, 40-65.
Alders, M, Winterhalder, D and Wessling, M (2017), “Helium recovery using membrane processes”, Separation and Purification Technology, 189 (2017), 433-440.
Bakhsh, MSA, Jaynes, SE, Neu, BT, Smolarek, J and Emley, T (2007), Helium Recovery.
Baksh, MSA (2010), “Methods and systems for helium recovery”, US patent no. 2010/0251892 A1.
Barboiu, C, Mourgues, A, Sala, B, Julbe, A, Sanchez, J, de Perthuis, S and Hittner, D (2006), Desalination 200, 89.
Bernado, P, Drioli, E and Golemme, G (2009), Ind. Eng. Chem. Res. 48, 4638.
Crawford, D, Coyle, DA and Anantharaman, B (2010), “Method for nitrogen rejection and or helium recovery in a liquefaction plant”.
Daly, JW (2005), “Helium recovery from LNG.” International Petroleum Technology Conference, American Association of Petroleum Geologists (AAPG); the European Association of Geoscientists and Engineers (EAGE); the Society of Exploration Geophysicists (SEG); and the Society of Petroleum Engineers (SPE), Doha, Qatar, 1–4.
Häussinger, P, Glatthaar, R, Rhode, W, Kick, H, Benkmann, C, Weber, J, Wunschel, HJ, Stenke, V, Leicht, E and Stenger, H (2005), “Noble gases”, in: Ullmann’s Encyclopedia of Industrial Chemistry, G. Walter, editor, Wiley-VCH Weinheim, Baden-Wärttemberg, Germany.
Helium One (2017), “Technical Presentation”, June 2017.
Hosseini, SS (2009), “Membranes and Materials for Separation and Purification Hydrogen and Natural Gas, Department of Chemical Engineering and Biomolecular Engineering, National University of Singapore.
Kim, D. (2014), “Helium Extraction form LNG End Flash”, Department of Energy and Process Engineering, Norwegian University of Science and Technology.
Li, X, Zhou, C, Lin, Z, Rocha, J, Lito, PF, Santiago, AS and Silva, CM (2011), Microporous Mesoporous Mater. 137, 43.
Lindemann, U, Boeck, S, Blum, L and Kurtcuoglu, K (2010), AIP Conf. Proc., 1218, 271.
Mehrpooya, M and Shafaei, A. (2016), “Advanced exergy analysis of novel flash-based helium recovery from natural gas processes”, Energy, 114 (2016), 64-83.
Ritter, JA and Ebner, AD (2007), Sep. Sci. Technol. 42, 1123.
Rufford, TE, Chan, IK, Huang, SH and May, EF (2014), “A Review of Conventional and Emerging Process Technologies for the Recovery of Helium from Natural Gas”, Adsorption Science & Technology, 32 (2014), 49-72.
Scholes, CA and Ghosh, UK (2017), “Review of Membranes for Helium Separation and Purification”, Membranes, 7, 9.
Scholes, CA, Ghosh UK and Ho, MT (2017), Indust. Eng. Chem. Res. 56 (2017), 335-383.
Scholes, CA and Ghosh, UK (2016), “Helium separation through polymeric membranes: Selectivity targets”, J. Membr. Sci. 520 (2016), 221–230.
Schrier, J. (2010), J. Phys. Chem. Lett., 1, 2284.
Seader, JD and Henley, EJ (2006), Separation Process Principles, John Wiley & Sons, Hoboken, NJ.
Soleimany, A., Hosseini, SS and Gallucci, F (2017), “Recent progress in developments of membrane materials and modification techniques for high-performance helium separation and recovery: A review”, Chemical Engineering & Processing: Process Intensification, June 2017, URL http://dx.doi.org/10.1016/j.cep.2017.06.001.
Stern, SA, Sinclair, TF, Hareis, PJ, Vahldieck, NP and Mohr, PH (1965), Ind. Eng. Chem., 57, 49.
Sunarso, J, Hashim, SS, Lin, YS and Liu, SM (2017), “Membranes for helium recovery: An overview on the context, materials and future directions”, Separation and Purification Technology, 176 (2017), 335-383.
Tagliabue, M, Farrusseng, D, Valencia, S, Aguado, S, Ravon, U, Rizzo, C, Corma, A and Mirodatos, C (2009), Chem. Eng. J. 155, 553.
Victory, D, Miles, MW and Oelfke, RH (2009), “Helium recovery from natural gas integrated with NGL recovery”, Google Patents.
West, JE (2009), “Helium extraction and production techniques.” URL: (Accessed: January 27, 2011).
This article explores aspects of seismic analysis of jetties used in the import/export of oil, gas, petroleum and other minerals. The design of jetties in offshore or nearshore waters in seismic active areas often poses challenges with structural analysis and design that other conventional structures do not have problems with. In this article, EPCM explores the conditions that govern the seismic analysis and design of marine jetties and gives some guidelines on how to address these governing conditions.
The terms “Pier” and “Jetty” are often used interchangeably, such as LNG Pier or LNG Jetty. Both refer to a fixed offshore or nearshore platform raised above the water, typically on piled foundations.
In the oil and gas industries, the term Jetty refers to the “Pier” type, i.e. a platform above water used to port oil, gas, minerals and other cargo. This article will use “Jetty” and focus on Jetties used as Port Terminals for Oil, Gas, and other Minerals in seismic active areas.
API 2A 2007 defines a seismic active zone as an area where the Peak Ground Acceleration (PGA) is more than 0.05g. In such cases, the design of the lateral resisting system (comprising the pile and deck frame) is typically governed by seismic actions.
Where PGA<0.05g, the structural pile and deck frame are typically governed by other environmental loads, such as extreme tropical storms with high waves, winds, currents or tsunamis.
The performance criteria for conventional buildings during seismic events are typically only to prevent catastrophic structural failure and not to prevent damage or to maintain functionality.
Due to the high economic costs of repairing marine structures and the loss of income during the shutdown, marine jetties typically have to withstand “frequently occurring” earthquakes without any damage or loss of functionality, ensuring they are immediately operational after such events. In extreme seismic events, the structures must withstand catastrophic collapse, although some degree of damage is permissible.
How does this translate into the analysis?
A jetty structure will have to be designed for two earthquake magnitudes.
For a “smaller, but statistically more frequent” seismic event, the structure must meet serviceability limit state criteria and remain fully elastic, with no plastic deformation allowed. These criteria are usually limiting:
For a “larger, but statistically less frequent” seismic event, the structure is allowed to behave plastically. This means that the structure can experience some damage, such as severe concrete cracking or spalling, and steel structures can form plastic hinges. Major repair works would be expected after such an event, and operations will typically be halted or limited until the structural integrity of the jetty structures is restored. Only ultimate strength is considered here.
Ultimate Limit State (ULS)
For the ULS, structural members may be designed to experience some damage, provided that catastrophic collapse is prevented. Structural members shall be designed using the seismic response spectrum appropriate for:
Seismic Analysis of Jetties: Serviceability Limit State
The structure shall be designed to limit deflections, crack widths, differential sway and other serviceability requirements. It shall remain perfectly elastic, with no plastic behaviour permitted in structural connections.
The seismic response spectrum used shall be for:
Distribution of Mass
During an earthquake, the ground accelerates, pulling the structure along with it. Because of the mass acceleration, inertial forces are induced:
Force = mass x acceleration
A unique feature of jetties and marine platforms is that their mass is concentrated at the top of the structure, whereas buildings have the mass distributed throughout the height of the structure. The mass is concentrated far from the soil embedment, causing greater deflections and stresses in the piles.
Loads Contributing to Seismic Mass
It is important to consider all loads that may contribute to mass acceleration during an earthquake. Live loads that may be present during an earthquake should be considered part of the seismic mass that causes horizontal forces.
Typically, loads to be considered as seismic mass:
To simplify the analysis of a piled structure, the piles can be modelled as if the structure were fully fixed (like a cantilever) at a depth referred to as the pile fixity.
Pile fixity values are typically taken between 3 and 7 pile diameters. For large pile diameters (D>1.2m) or stiffer soils, lower values of 2D-4D can be used, but a conservative value of 6D is often used for the initial design.

Scouring around the pile can be expected due to ocean bottom currents or streams caused by vessel propellers.
If the pile fixity is in the top layer of soil, potential loss of support due to scouring should be considered. Scouring can range from 0.5 m to 1.0 m.

Saturated soils may lose their stiffness and strength and take liquid-like properties due to the vibration of the soil during seismic events.
Any liquefiable layer of soil will not provide sufficient support for piles, and the structure should be modelled such that piles are fixed adequately in a non-liquefiable layer of soil.
Pile fixity should not be located too close to a liquefiable layer of soil to prevent the risk of punching through this layer during an earthquake. As a general rule of thumb, if the pile is fixed within 3-5 diameters above a liquefiable layer, it is advisable to position the pile fixity below the liquefiable layer.
Fixing the piles at a lower level means piles experience more stress due to horizontal loads, and deflections may increase due to longer unsupported pile lengths.

Loading platforms, access trestles and other jetty structures that house sensitive equipment or piping are subject to strict deflection criteria that often govern the structural layout and sizes of piles in seismic active zones. The lateral bearing pressure of the soil is critical for seismic design, but pile lengths are usually only governed by normal static conditions.
Overall horizontal deflection is usually limited between H/400 and H/200, where H is the height from the point of fixity to the top of the deck. For access trestles, overall longitudinal deflections are limited to 200mm, and overall lateral deflections to either span/225 or 100mm. For trestles housing pipes, deflections may even be limited to span/500 to prevent damage to pipes (often containing high explosive products).
For areas of high seismicity, these strict deflection criteria structures often lead to pile sizes of jetty loading platforms and access trestles that are much larger than required for strength criteria.
A way of curbing horizontal deflections could be to use a stiffer structure by introducing raked piles and/or larger diameter piles. However, a stiffer structure may experience higher loads during an earthquake, potentially leading to an iterative design process until a structural layout can meet these serviceability requirements.
During an earthquake, the fundamental period of a structure (a measure of its slenderness/stiffness) affects how much acceleration, therefore force, a structure experiences during an earthquake.
Slender structures (higher structural periods), such as masts, steel towers or high-rise steel buildings, may experience far less force than rigid structures (lower structural periods), such as concrete buildings. Although slender structures experience less force, they deflect more.
Increasing the stiffness does not always sufficiently reduce deflection in an earthquake, because the load increases as the stiffness does.
The design of the structural layout of a jetty needs to find a balance between:
Loss of thickness in the steel piles needs to be considered for seismic analysis. The strength of steel piles should be based on a reduced wall thickness, but the analysis should consider the full mass of the pile cross-section.
Typical corrosion rates for structural steel in the splash zone are 0.01-0.03mm/year. Typical rates for design are given by BS-6349 or EN 1998.
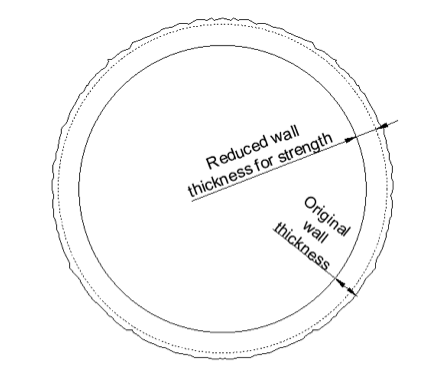
Figure 2: Loss of Thickness due to Corrosion
An earthquake can occur in any direction, and due to the “shaking” action, loads are reversible. Therefore, all possible directions of seismic action should be considered in the structural analysis of the jetty.
It is common in access trestles for raked piles to be orientated in the lateral direction only. The deflection criteria for lateral movement are often stricter than those for longitudinal movement, and longitudinal load is shared between a few pile groups.
This means that the structure is stiffer in the lateral direction than the longitudinal direction. When analysing the seismic event with the dominant direction in the longitudinal direction, the seismic acceleration will be less on the structure because the structure has a higher period of vibration in that direction.
For example, when analysing the forces in the lateral direction of an access trestle, 100% of the seismic activity is used in the lateral direction and 30% in the longitudinal direction. This seismic acceleration based on the stiffness of the lateral direction should be used for this case. When the dominant direction is longitudinal, 100% of the force based on the longitudinal stiffness is applied to the longitudinal direction, and 30% in the lateral direction.

The seismic analysis shall consider the following combination:
(1.0 +/- 0.5PGA)D + 0.3L + 1.0E
Where,
D = loads, such as the weight of the structure, fixed equipment, any mass that may be present during an earthquake and loads that occur more than 50% of the time
L = Live Loads: 30% of transient live loads not considered as part of the seismic mass
E = Horizontal earthquake loads as calculated (use D in the calculation to determine seismic mass)
PGA = peak ground acceleration
Berthing, mooring and environmental loads (wave, wind and current) need not be considered in combination with the seismic action.
For more detailed guidelines on seismic analysis, refer to the literature below:
Seawater intake and outfall systems are used worldwide to draw in seawater and disperse brine for desalination and power plants. These systems produce potable water for drinking and serve as a source of cooling and steam to aid onshore processes.
The following are the main items of seawater intake and outfall system:

Overview of pipeline structure
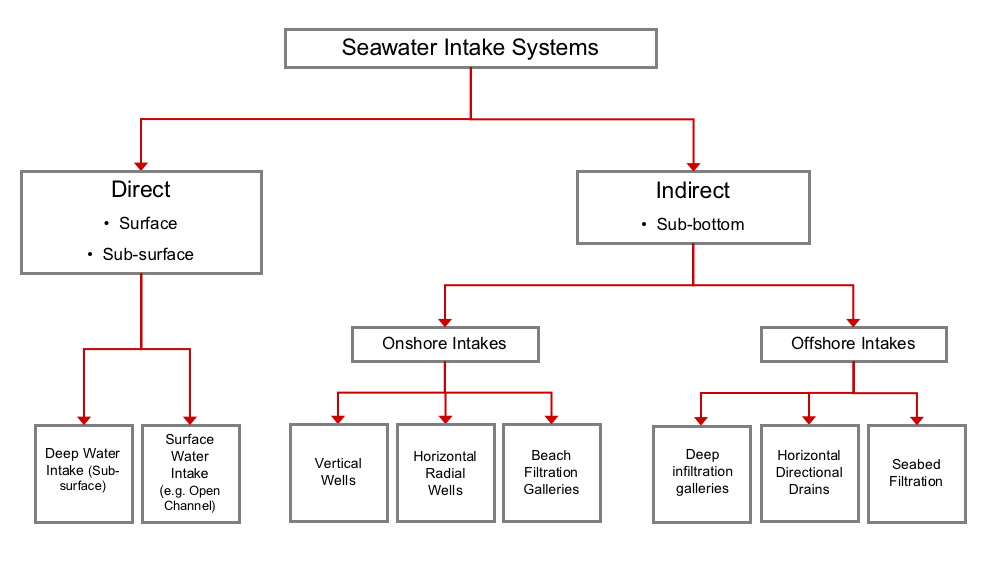
Seawater Intake & Outfall Systems

Plan view of surface intake

Side view graph of sub-surface intake
For shallow outfalls (typically less than 20m water depth), the diurnal land and sea breezes will result in diurnal changes in the surface waste fields’ transport (onshore/offshore).
Water levels and associated currents govern the hydraulic design of the intake and outfall, as well as the dispersion and transport of the effluent plumes.
Waves are a critical environmental factor essential for the design and construction of marine structures.
Initial and secondary dilutions.
Stratified conditions, characterized by layering in the water column due to a density gradient between the surface and the bottom, can prevent a buoyant plume from rising, leading to reduced initial dilution and the formation of a submerged waste field beneath the surface. However, since the effluent to be discharged offshore is denser than the surrounding seawater, the potential impact of stratification on initial dilution is considered negligible.
Larger marine life is trapped in or against the intake screens in the intake openings due to the velocity and force of the water flowing through them.
Very small and microscopic organisms (e.g. phytoplankton, zooplankton, eggs and larva) are pulled through the screens and into the abstraction system.
Measures to reduce impingement and entrainment associated with direct intake systems – recommended by the United States Environmental Protection Agency (EPA 1985, 2001 and 2004 DESALINATION ISSUES ASSESSMENT REPORT 2003 and SYNTHESIS PAPER )
The balance between proximity to desalination plant versus environmental & physical requirements & characteristics.
Generally, the deeper the intake structure (furthest offshore) and with the extraction point raised a few meters above the seafloor, the cleaner the feedwater tends to be, as it typically contains less sediment in suspension. This setup also reduces the impact of wave forces on the structure while ensuring that the extraction point remains submerged at all times.
The primary treatment processes at the plant increase in complexity and cost as the feedwater quality decreases. However, the further offshore the extraction location, the more expensive the initial construction costs.
The location of existing ocean outfalls should be considered, as this could affect the feedwater quality, particularly the location of the brine outfall, which could lead to re-circulation.
From an environmental point of view, for a subsurface direct offshore intake, the deeper the extraction point is located, the less oxygen in the water and, subsequently, marine life. When selecting a location for a direct surface intake at the shore, the beneficial uses, environmentally sensitive areas and possible negative effects on the coastline (alongshore sediment regime) have to be considered.
Finally, the proposed location’s proximity to ports and fishing activities, together with popular navigation ship routes, should be taken into consideration. Ship anchors can cause major damage to subsurface structures, fishing nets can block the intake screens, and pollution caused by vessels or port activities could impact feedwater quality.
The following design guidelines, which are specified in the Coastal Engineering Manual (EM 1110-2-3001, 1995), should be taken into account to ensure the optimum hydraulic performance of a seawater intake structure:
The intake structure should be designed to withstand wave stresses and current forces on the intake head during adverse sea conditions. Provision should be made for safe navigation of seafaring vessels.

Seawater Intake & Outfall Systems
The effluent discharge system is designed to minimize environmental impact while ensuring full compliance with relevant environmental guidelines, regulations, and legislation.
It is always better to discharge continuously.
When fluctuations occur due to unforeseen production or operational issues, the outfall system should be designed to discharge intermittently at design flow rates.
The outfall system is designed to comply with environmental criteria and hydraulic requirements for a specific design flow rate and effluent composition. Therefore, the system will not perform according to the design (environmental and physical) requirements if the effluent is discharged at a reduced flow rate.
The term dilution describes the process of reducing the concentration of effluent constituents by mixing the effluent with uncontaminated ambient seawater. This process aims to achieve acceptable concentration levels that support ecosystem functioning and allow for safe recreational human activities, such as swimming. The required dilution is a function of the effluent concentration and the “buffer capacity”, which is the difference between a guideline value (target value) and the ambient concentration of the specific water quality variables.
The required initial dilution for the concentration of conservative constituents can be estimated by the conservation of mass as follows (DWAF, 2004):
S = (Ce – Cb) / (Cg – Cb)
Where:
S = Required dilution
Ce = Concentration of constituent in wastewater
Cb = Concentration of constituent in receiving marine environment (ambient concentration)
Cg = Recommended concentration (guideline)
The total dilution of conservative constituents at a distant location can be considered as two distinct processes: the initial dilution when the effluent stream is injected into the receiving water body, and the secondary dilution where the waste field is transported to a distant location.
For an offshore outfall (deep water), the initial dilution is brought about by the entrainment of clean seawater when effluent is jetted out into the receiving water body. The degree of entrainment is related to the shear between the plume and the adjacent water, a function of the effluent jet’s momentum and buoyancy. The initial dilution process will cease when the plume’s vertical velocity reaches zero, or the plume reaches the water’s surface.
The effluent field will then be further diluted by diffusion (eddy) while being transported away by ocean currents—secondary dilution. The vertical behaviour of the effluent plume will be affected by layering (stratification) in the water column, depending on the effluent’s relative density relative to the density of the receiving water body.
The physical properties of brine plume (negatively buoyant) limit the initial dilutions which can be achieved. Dense plume will sink to the seafloor.

Outfall Systems

Seawater Intake & Outfall Systems
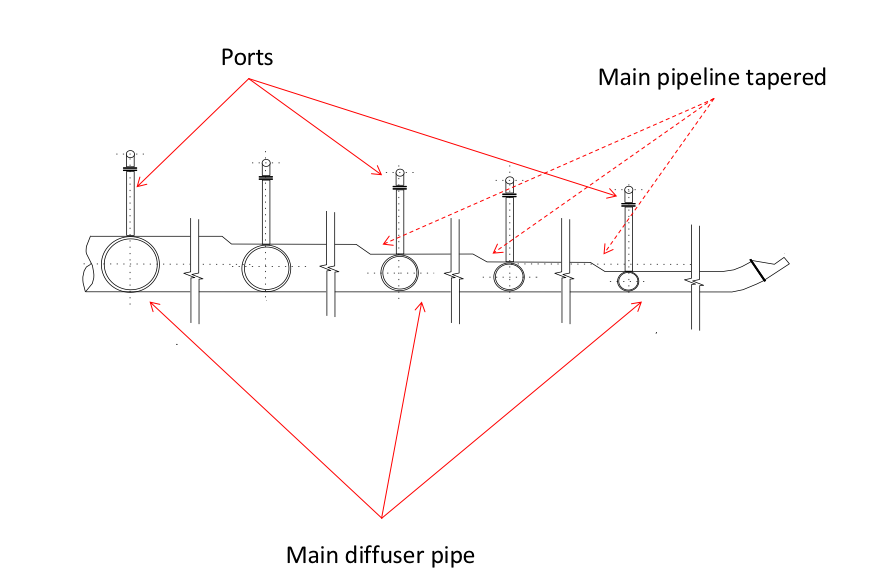
Seawater Intake & Outfall Systems
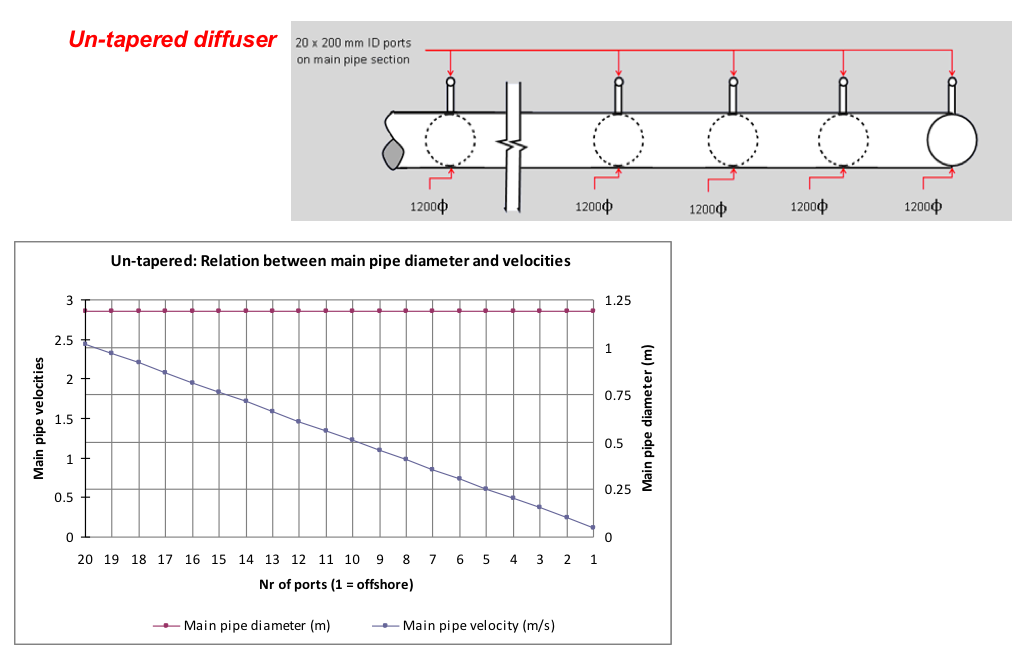
Seawater Intake & Outfall Systems
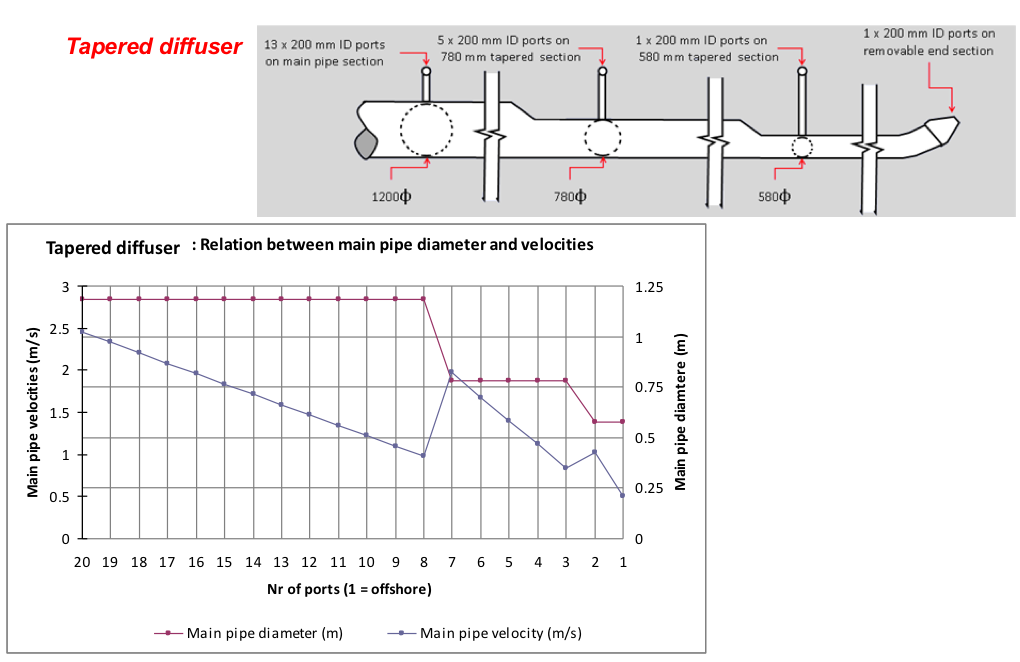
Seawater Intake & Outfall Systems
To optimize the diffuser configuration, consider the following influencing aspects (controlling parameters):
Effluent density: which is denser than seawater for a brine effluent.
The required dilution necessary to meet the environmental objectives is typically around 20, depending on site-specific requirements.
The diameter of the main pipeline, initially determined for the outfall pipe, will determine the diffuser configuration, including port diameters and the number of ports.
Additional momentum is needed to ensure the jet plume has a long enough path to entrain seawater and achieve the required dilutions. Therefore, the momentum flux for each port must be increased. However, it is advisable not to raise port velocities too high, as increased velocities can lead to greater forces on diffuser components.
Although a brine diffuser does not require great depth due to the limiting rising height of the effluent plume, more inshore marine structures are more vulnerable to nearshore physical processes, such as wave forces and unstable seabed conditions.
The discharge angle of the ports should be inclined relative to the horizontal to maximize the plume’s path length.
Initial modelling, using numerous configurations, is required to optimize the diffuser design.
To provide developers with an initial estimate of the diffuser configuration requirements, a method was developed based on scientific principles and widely accepted environmental regulations. This approach offers a rough idea of the required number of ports for a specific discharge flow rate and port diameters.
Numerous prediction theories and techniques are available. The choice of the technique (“model”) to be applied is to be decided upon by the design engineer, taking the following into account:
Not one of the theories/ prediction techniques available can be considered inaccurate because they were not developed in isolation but were part of the “evolution” of an overall concept, supported and verified by numerous field and laboratory experiments.
The essential issue is that the user of any “model” must be fully aware of the estimation’s sensitivity to the complexity of the continuously varying processes in the receiving environment.

Seawater Intake & Outfall Systems
| µg/L (micrograms per litre) | Micrograms per litre; a measurement describing the amount of a substance (such as a mineral, chemical or contaminant) in one litre of water. It is expressed in terms of weight per volume. One µg/L is equal to one part per billion. |
| Beneficial use area | Desired uses of the marine and estuarine areas |
| Biocide | A chemical (e.g. chlorine) used to kill biological organisms. |
| Brine | Water that contains a high concentration of salt. Brine discharges from desalination plants may also include constituents used in pre-treatment processes, in addition to the high salt concentration seawater. |
| Bromide | An element that is present in desalinated seawater. |
| Coagulation | A pre-treatment process used in some desalination plants. A substance (e.g., ferric chloride) is added to a solution to cause certain elements to thicken into a coherent mass, so that they may be removed. |
| Coastal area | The area where the land is influenced by its proximity to the sea, and where the sea is influenced by its proximity to the land. |
| Cogeneration | A power plant designed to conserve energy by using “waste heat” from generating electricity for another purpose. |
| Concentrate | Water that contains a high concentration of salt. Concentrate discharges from desalination plants may include constituents used in pre-treatment processes, in addition to the high salt concentration seawater. |
| Conventional treatment | A method of treating water which consists of mixing, coagulation-flocculation, sedimentation, filtration, and disinfection. Similar to direct filtration with the addition of flocculation and sedimentation. |
| Deaeration | Removal of oxygen. A pre-treatment process in desalination plants to reduce corrosion. |
| Desalination | Desalination is the process of removing dissolved salt and other minerals from seawater to create fresh water. |
| Diffuser | The offshore component of an outfall system, consisting of the main pipe (with or without tapers) equipped with discharge ports placed at specific intervals. It is designed to provide an even distribution of port flows along with the diffuser. |
| Dilution | The lessening in the concentration of a substance due to mixing with water. |
| Direct seawater intake | Open water intake extracts water directly from the sea. |
| Discharge | A return stream from the desalination plant that is released back into the environment through dilution and mixing. |
| Disinfection | Water treatment which destroys potentially harmful bacteria. |
| Distillation | A process of desalination where the intake water is heated to produce steam. The steam is then condensed to produce product water with low salt concentration. |
| Ecosystem | A community of plants, animals, and organisms interacting with each other and their environment’s non-living (physical and chemical) components. |
| Eddies | The movement of a stream of water in which the current doubles back on itself, causing a type of “whirlpool”. This is typically caused by promontories along a coastline or counteractions from driving forces such as wind shear and an ambient current. |
| Electrodialysis | Most of the impurities in water are present in an ionized (electrically charged) state. When an electric current is applied, the impurities migrate towards the positive and negative electrodes. This leaves the intermediate area with reduced impurity levels, resulting in a purified stream of product water. While effective for treating brackish water, this technology is not yet used commercially for desalting seawater on a large scale. |
| Environmental impact | A positive or negative environmental change caused by human action. |
| EPA | The United States Environmental Protection Agency |
| Estuary | A partially or fully enclosed body of water open to the sea permanently or periodically, and within which the seawater can be diluted, to a measurable extent, with freshwater drained from land or a river. The upstream boundary of an estuary is the extent of tidal influence. |
| Euphotic zone | The depth of the water body in an ocean that is exposed to sufficient sunlight for photosynthesis to occur. |
| Far-field dilution | As an effluent plume moves away from the initial mixing zone, it undergoes secondary dilution through processes such as dispersion, entrainment, and mixing with seawater. This dispersion is driven by factors like currents, turbulence, eddies, and shears. Additionally, the chemical and biological dispersion of non-conservative substances and the decay of certain organisms contribute to what is known as the “far-field dilution process”. |
| Feedwater | Water fed to the desalination equipment. This can be source water with or without pre-treatment. |
| Filtration | A process that separates small particles from water by using a porous barrier to trap the particles and allow the water through. |
| GRP | Glass Reinforced Polyester/Plastic |
| HDPE | High-Density Polyethylene |
| Head loss | The drop in the sum of pressure head, velocity head, and potential head between two points along a path. |
| Hydraulic grade line (HGL) | The height to which the water would rise in a piezometer tube attached vertically to the water conveyance pipeline. |
| Indirect seawater intake | Intake water filtered through the seabed (e.g. via beach wells). |
| Infiltration Gallery | A method used for seawater intake. Perforated pipes are arranged in a radial pattern onshore sand below the water level. Water in the saturated sand enters the perforated pipes. |
| Initial dilutions for dense plume | The dilution of the wastewater plume generated by jet momentum and the negative buoyancy effect that occurs, causing the plume to descend on the seabed. |
| Initial dilutions for buoyant plume | The dilution of the wastewater plume generated by jet momentum and the positive buoyancy effects that occur between the outlet ports of a marine outfall’s diffuser and the sea surface. |
| Intake | The physical facilities through which the seawater enters the plant. |
| Marine discharge | Discharging wastewater to the marine environment either to an estuary or the surf zone or through a marine outfall (i.e. to the offshore marine environment). |
| MarineEnvironment | The marine environment includes estuaries, coastal marine and near-shore zones, and open-ocean-deep-sea regions. |
| Marine outfall pipeline | A submarine pipeline originating onshore conveys wastewater from a headworks to a submerged discharge location on or near the seabed beyond the surf zone (i.e. to the offshore marine environment). Also referred to in the literature as a long sea outfall/pipeline and ocean outfall/pipeline. |
| Mean sea level | The average elevation of the sea surface for all stages of the tides over a long period. |
| Membrane desalination | The use of membranes to remove salts from seawater. |
| Meteorological conditions | The prevailing environmental conditions as they influence the prediction of weather. |
| mg/L | Milligrams per litre is a measurement describing the amount of a substance (such as a mineral, chemical or contaminant) in one litre of water. One milligram per litre is equal to one part per million. |
| Micro-filtration (MF) | A water filtration method utilizing a pressure-driven membrane process that incorporates particle filters capable of rejecting particles larger than 1.0 microns. This process produces a less refined effluent compared to ultra-filtration. |
| Micro-layer | The upper few millimetres of the ocean. Fish eggs are sometimes concentrated in the micro-layer. |
| Mitigation | Preventing damage or repairing an area after construction or creating environmental improvements (sometimes in a different location). |
| Multi-effect Distillation (MED) | A form of distillation. Evaporators are in series, and vapour from one series evaporates water in the next. This technology has several forms, one of the most common of which is the Vertical Tube Evaporator (VTE). |
| Multi-stage Flash Distillation (MSF) | A form of distillation. The intake water is pressurized and heated. It is discharged into a chamber maintained slightly below the saturation vapour pressure of the water, and a fraction of the water content flashes into steam. The steam condenses on the exterior surface of heat transfer tubing and becomes product water. The unflashed brine enters a second chamber, where the brine flashes to steam at a lower temperature. Each evaporation and condensation series is called a stage. |
| MWQG | Marine Water Quality Guidelines |
| Nearshoredischarge | Diluting and mixing the concentrate with a large water flow and returning it to the nearshore area. |
| Ocean Thermal Energy Conversion (OTEC) | A solar, ocean thermal desalination approach where electricity is produced by using the temperature differential between cold, deep waters and warm, shallow surface waters. Water at the ocean surface (at about 70°F) is used to heat liquid ammonia, which vaporizes at this temperature in a vacuum chamber. The ammonia vapour is used to turn a turbine to produce electricity. The vapour is then condensed using cold water pumped up from the ocean depths (at about 35°F). |
| Offshore | Within the context of ocean outfalls, this is the zone in the sea in which wave action has an insignificant effect on water circulation and shoreline processes (erosion and accretion). |
| Offshore discharge | Discharge to the offshore areas. |
| Pollution | The direct or indirect alteration of the physical, chemical, or biological properties of the natural environment, including marine environments. This alteration makes the environment less suitable for its intended beneficial purposes or renders it harmful, or potentially harmful, to human health, safety, and welfare, as well as to aquatic and non-aquatic organisms. |
| Potable | Water that does not contain pollution, contamination, objectionable minerals or infective agents and is considered safe for domestic consumption. |
| PP | Polypropylene |
| Product Water | The desalinated water delivered to the water distribution system. |
| Reverse Osmosis (RO) | A desalination process where pressure is continuously applied to the feedwater, forcing water molecules through a semi-permeable membrane. Water that passes through the membrane leaves the unit as product water; most dissolved impurities remain behind and are discharged in a waste stream. |
| Rhodamine-B dye | A fluorescent red basic xanthene dye used in the marine environment to determine transport and dispersion patterns. |
| Saline water | Water that contains a significant concentration of dissolved salts (NaCl). |
| Salinity | Generally, the concentration of mineral salts dissolved in water. Salinity may be measured by weight (total dissolved solids – TDS), electrical conductivity, or osmotic pressure. Where seawater is known to be the major source of salt, salinity is often used to refer to the concentration of chlorides in the water. |
| SDI | Swartz’s Dominance Index is used to evaluate benthic community assemblages and is defined as the minimum number of species comprising 75% of the total abundance in a given sample. |
| Secondary dilutions | The further dilution that occurs after initial dilution when a wastewater plume is transported away from the discharge area. |
| Stagnant stratified conditions | The absence of currents and with stratification of the seawater (density gradient between the surface and the bottom). |
| Stagnant un-stratified conditions | The absence of currents and with no stratification of the seawater. |
| Stratification | When denser seawater underlies lighter seawater causing a vertical density gradient in the water column, depending on the vertical temperature gradient between warmer upper water layers and colder deeper water layers and the salinity gradient. |
| Surf zone | Also referred to as the “breaker zone”, where water depths are such that the incoming waves collapse and breakers are formed. |
| Suspended solids (SS) | Small solid particles that remain in suspension in water as a colloid or due to the motion of the water. It is used as one indicator of water quality. Sometimes abbreviated as SS, it should not be confused with settleable solids, also sometimes abbreviated SS, which contribute to the blocking of sewer pipes. |
| Seawater Reverse Osmosis (SWRO) | A desalination process where pressure is continuously applied to seawater, forcing water molecules through a semi-permeable membrane. Water that passes through the membrane leaves the unit as product water; most of the dissolved impurities remain behind and are discharged in a waste stream. |
| Thermaldesalination process | It involves heating seawater, generating water vapour, which is then condensed to produce fresh water. |
| Total Dissolved Solids (TDS) | Total salt and calcium carbonate concentration in a sample of water, is usually expressed in milligrams per litre (mg/L) or parts per million (ppm). The state- recommended Maximum Contaminant Level (MCL) drinking water standard for total dissolved solids is 500 mg/L, the upper MCL is 1,000 mg/L, and the short-term permitted level is 1,500 mg/L. |
| Total dynamic head | The summation of the hydraulic head (elevation, pressure, and/or friction losses) that the flow of water must overcome to move forward. |
| TSS | Total Suspended Solids |
| Turbidity | A measure of suspended solids concentration in water. |
| Ultra-filtration(UF) | A membrane filtration process that falls between RO and MF in terms of the size of particles removed. |
| Ultraviolet Treatment (UV) | The use of ultraviolet light for disinfection. |
| Vacuum Freezing (VF) | A process of desalination where the temperature and pressure of the seawater are lowered so that the pure water forms ice crystals. The ice is then washed and melted to produce the product water. This technology is still being developed and is not yet commercially competitive. |
| Vapour Compression | A form of distillation. A portion of feedwater is evaporated, and the vapour is sent to a compressor. Mechanical or thermal energy is used to compress the vapour, which increases its temperature. The vapour is then condensed to form product water, and the released heat evaporates the feedwater. |
Discussed in this report are the characteristics of and comparisons between Single Point Mooring (SPM) systems and Conventional Buoy Mooring (CBM) systems, also referred to as Multi-Buoy Moorings (MBM). These systems are used for short term mooring applications associated with the offloading and loading of bulk liquid fuel tankers transporting refined and unrefined products.
Permanently moored vessels are excluded from the scope.
(See our full detailed article on SPM CALM Systems)
An SPM is a loading buoy anchored offshore that serves as a mooring point as well as an interconnection for tankers loading or offloading liquid products.
The tanker is moored at the bow by means of one or two hawsers to the buoy. The vessel always takes the most favourable position in relation to the combination of wind, current and wave and is free to align itself with the prevailing environmental forces at the time. As the vessel in its stationary state is always positioned head-on into the winds/currents direction, the total force is less than would be experienced by a vessel on a fixed mooring which is not always head-on into the prevailing conditions.
The vessel will approach the buoy with its bow into the dominant environment, thus maximising control while minimising the need for tug assistance.
A tug is required at all times during mooring and offloading to maintain the nominal amount of tension on the mooring hawsers to prevent collision of the tanker with the buoy and assist with the weather vane of the vessel.
The fluid transfer system includes submarine hoses between the pipeline end manifold (PLEM) on the seabed and the buoy and floating hoses between the buoy and the tanker. The buoy contains a swivel that provides the fluid transfer path between the geostatic part and the rotating part of the buoy.
Refer to Figure 1 illustrating a vessel moored to an SPM system.
The SPM system consists of four main components:

Figure 1: SPM system including moored vessel and connected floating hoses
The buoy body is held in place by means of static legs attached to the seabed underneath the surface. The body has a rotating part above water level connected to the offloading/loading tanker. These two sections are linked by a roller bearing, referred to as the Main Bearing. The moored tanker can freely weathervane around the buoy and find a stable position due to this arrangement.
The type of bearing used and the split between the rotating and geostatic parts determine the concept of the buoy. SPM mooring concepts are distinguished in Table 1.
The size of the buoy is a function of the counter buoyancy needed to hold the anchor chains in position, and the chains are a function of the environmental conditions and the vessel size. Figure 2 illustrates a Turntable type SPM system during installation.
Moorings fix the buoy to the seabed. The type of anchoring system is greatly dependent on local soil conditions and may consist of ships-, rig-, piles- or gravity anchors.
The two main types of mooring configurations for SPMs are Catenary Anchor Leg Mooring (CALM) and Single Anchor Leg Mooring (SALM). CALM holds the buoy in place by anchor chain that extends in catenaries to anchor points some distance from the buoy.
The SALM system is similar, except that the SALM is anchored by a single anchor leg. The primary benefit of a CALM Buoy over a SALM Buoy is ease of maintenance. The vast majority of Marine Terminals installed since the mid-1990s have been CALM Buoys. Figure 3 illustrates the CALM Buoy type concept.
Mooring components of a CALM system:

Figure 2: SPM (Turntable) being towed into position

Figure 3: SPM CALM Buoy system configuration
The heart of each SPM is the product transfer system. This system transfers products from the tanker to the PLEM located on the seabed.
The basic product transfer system components are:
Other possible components of SPMs are:
There are no vessel constraints for an SPM. The SPM CALM buoy configuration can accommodate the largest vessels, including VLCCs.
SPM CALM buoy systems are suited for locations up to approximately 100 m water depth. This is preferential when deep waters are not available close to shore.
The minimum depth is a constraint for the design vessel Under Keel Clearance (UKC).
Conventional Buoy Mooring (CBM) or Multiple Buoy Mooring (MBM) is a mooring facility consisting of three to eight permanent mooring chains with anchors. It holds the vessel in a fixed position and does not allow it to weathervane. A buoy is attached to the end of each chain to mark the position of the chain and allow pickup of the chain by the vessel to be moored.
The number of mooring chains is a function of the vessel size, metocean conditions and navigational constraints.
As soon as the tanker has manoeuvred into position with the aid of tugs, a launch crew takes the tanker lines, one at a time, and tows them to the various mooring buoys.
When berthed, the tanker remains in a fixed position without tugs and depending on the site-specific conditions, sometimes without vessel anchors.
The ships’ mooring ropes are connected from either side of the vessel from the bow and the stern to quick release hooks on the conventional buoys.
After mooring the tanker to the buoys, pickup of the submerged hose strings and connecting the hoses to the midship manifold, the loading or offloading operation may start. The other end of the hose string is connected to the PLEM.
The tanker needs assistance in disconnecting from the mooring and navigating away from the CBM system. Figure 4 is a representation of a general CBM system.

Figure 4: CBM system (four conventional buoys)
The buoys in a CBM system are conventional in that they act as mooring only structures. They contain no product transfer paths.
The size of the buoys is a function of the counter buoyancy needed to hold the anchor chain for each buoy in position.
The buoys are manufactured either from steel or polymer materials.
The mooring system comprises of Mooring Buoys and Mooring Legs, where the buoys are generally moored to the seabed with chain legs and high capacity anchors, piles or clump weights depending on soil characteristics.
A typical CBM system’s buoys have mooring assemblies through the centre of the units, terminating in a mooring eye on the bottom and padeyes on top for the fitting of quick release hooks
The mooring legs for each buoy only consists of one anchor, unlike in the case of a CALM system where the buoy has between six and eight anchors. This is because the anchor chains of a CBM system only needs to work in one direction.
When the system is not in use, the hose string is laid down on the seabed away from the influence of the waves. The end of the hose is provided with a pick-up line and a marker buoy. The hose string is picked up by a small support vessel that also assists the tanker with mooring to the buoys. CBMs can be operated with up to four separate product lines
As no weathervaning is possible, it is often applied on projects where smaller tankers are employed, in areas where the environmental conditions are moderate or uni-directional and where the frequency of loading/offloading operations is limited.
The system can be designed to berth all sizes of tankers, with the largest CBM system in the world designed to accommodate vessels up to 225 000 dwt (Puma multi-directional loading facility, Angola, 2015).
CBM systems are ideally suited to shallow water applications up to approximately 30m water depth, in benign environmental conditions or conditions with a dominant directional character.
The minimum depth is a constraint of the design vessel UKC.
The offloading/loading operations can be undertaken via a number of hoses (one to four hoses) and can handle multiple products if required.
| CRITERIA | SPM | CBM |
| Vessel size | Vessel sizes unlimited | Vessel sizes typically up to 80 000 dwt, but up to 225 000 dwt has been installed |
| Operating water depth | Up to 100 m water depth | Up to 30 m water depth |
| Mooring chains | 6 to 8 mooring chains | 4 to 8 mooring chains |
| Approach | Can approach from any position – and therefore can choose to approach into prevailing weather conditions | Can only approach from limited positions |
| Mooring time (after arriving at mooring position) | Typically 15 min. | Typically 2.0 h |
| Hose connection time | Typically 1.0 h | Typically 1.0 h |
| Offloading time | The function of vessel pumps capacity, line size and distance | The function of vessel pumps capacity, line size and distance |
| Hose disconnection time | Typically 1.0 h | Typically 1.0 h |
| Mooring disconnection time | Typically 15 min. | Typically 1.0 h |
| Net total time difference | Typically 2.5 h quicker compared to CBM | Typically 2.5 h longer compared to SPM |
| Mooring conditions | Able to moor with winds up to 30 knots and head waves of 2.0 m to 2.5m | Able to moor with winds up to 30 knots and head waves of 1.0 m |
| Offloading steps | Steam from anchorage, moor, connect hoses, pump product through, disconnect hoses, disconnects mooring, steams away | Steam from anchorage, moor, connect hoses, pump product through, disconnect hoses, disconnects mooring, steams away |
| Operating limit | Product offloading possible with wind up to 40 knots and head waves of 3.0 m to 4.5 m | Product unloading with wind up to 40 knots and waves higher than 2.0 m to 2.5 m |
| Mooring disconnection | The vessel has to leave berth with winds of 60 knots and waves higher than 3.5 m to 5.0 m | The vessel has to leave berth with winds of 60 knots and waves higher than 2.0 m to 3.0 m |
| Cost | Approximately US $15m for SPM CALM buoy alone | Approximately US $120k to $ 250k per buoy alone |
| Tuggage | Required for mooring. Tug required full time during mooring to assist with weathervane movements | Required during mooring and disconnection from moorings |
| Under Keel Clearance | The function of the vessel and metocean conditions | The function of the vessel and metocean conditions |
| Suppliers | Proprietary technology and limited suppliers | Non-proprietary technology |
| Track record | Since 1959 successfully installed around the world | Successfully installed around the world for close to a century |
| Weather impact | Less prone to adverse conditions and swell delays than a CBM | More prone to adverse conditions and swell delays than an SPM |
| Night operations | Possible limitation on night-time mooring depending on local operational, safety and environmental procedures. Can disconnect from moorings 24 h a day | Possible limitation on night-time mooring and disconnecting from mooring depending on local operational, safety and environmental procedures |
| Maintenance | Additional complex maintenance activities compared to a CBM – swivel, bearings, mooring line tensions, etc. | Less complex maintenance compared to SPM. Hoses damage more due to abrasion from storage on the sea bed |