1 Introduction
The combustion of fossil fuels is acknowledged as the single largest anthropogenic source of Carbon Dioxide emissions. The capture of Carbon Dioxide from Flue gases is an important part of the transition roadmap to a low Carbon, sustainable energy future.
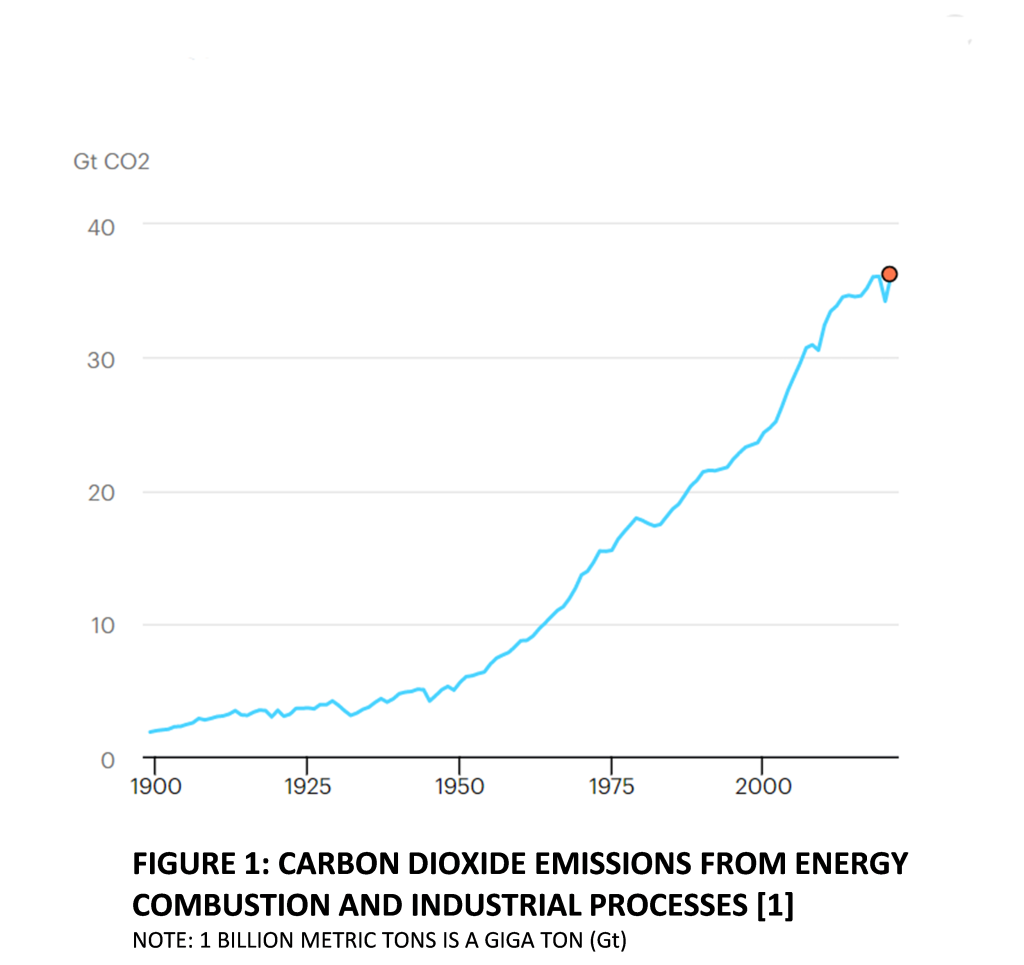
During the year 2021, an estimated 36.3 Gigatons (Gt) of Carbon Dioxide were emitted from fossil fuel combustion in the global energy and industrial process sectors. There is a direct correlation between GDP growth rates and Carbon Dioxide emissions from the energy and industrial sectors. This is apparent from Figure 1 which displays historical Carbon Dioxide emission trends from these sectors and shows a dip in emissions during the 2020 pandemic induced slowdown, followed by a sharp increase as GDP recovered to pre-COVID levels [1].
Globally, Carbon Dioxide constitutes more than three-fourths of all green-house gas emissions [2]. Further, a quarter of all global Carbon Dioxide emissions are from the power generation and industrial sectors [3]. These post-combustion emissions are termed Flue gas emissions, as they emanate from exhaust stacks or chimneys of equipment such as Steam Boilers, Fired-heaters, Furnaces, Gas Turbines, Gas Engines and Liquid Fuel Engines.
In fossil-fuel based power plants, Coal, Furnace Oil, Diesel, and Natural Gas provide the primary thermal energy to drive electricity generation equipment. Coal is the most widely used fuel at present, but there is global consensus on phasing it out and the transition to cleaner fuels is underway.
In the industrial sector, fossil-fuels are used to provide process heat in Chemical, Metallurgical, and Mineral transformation processes. Among manufacturing industries, the following large-scale, energy intensive processes are major emitters of Carbon Dioxide from Flue gases:
- Iron and Steel
- Crude Oil refineries
- Fertilizer
- Petrochemicals
- Cement
Hence these are considered priority sectors and receive global attention for technology development and deployments relating to Carbon capture, utilization, and Sequestration.
2 Mitigation Strategies for Combustion Generated Carbon Dioxide
Currently there are three technological approaches to mitigate Carbon Dioxide emissions from Flue gases [4].
- Capture of Carbon Dioxide Post-combustion with Air:
In this approach Carbon Dioxide is captured from Flue gases leaving the stack. This is an end-of-pipe approach and generally does not involve changes to the existing process. However, apart from Carbon Dioxide and Water, the Flue gases contains Nitrogen and Oxygen from combustion Air, besides Oxides of Sulphur and Nitrogen and other components depending on the fuel.
- Pre-combustion capture:
This method converts fossil fuels to Syngas, with Carbon Dioxide being removed before fuel combustion. The fuel that is eventually combusted is either Hydrogen or a Hydrogen-rich mixture with Carbon Monoxide.
- Oxy-combustion (capture of Carbon Dioxide Post-combustion with Oxygen):
This involves reduction of Flue gas volume by eliminating the entry of Nitrogen via combustion Air. An Air separation plant generates pure Oxygen for fuel combustion.
Figure 2 illustrates the three approaches.

Of the three approaches, Post-combustion capture of Carbon Dioxide is the easiest to implement on existing facilities. This is because it minimizes disruption to existing processes. Post-combustion capture projects can be executed as brownfield retrofits and scheduled to coincide with plant maintenance shutdowns.
The second approach of Pre-combustion capture avoids Flue Gas Carbon Dioxide emissions during combustion. The idea is to achieve a fuel switch from fossil fuel to Hydrogen, by removing Carbon from the fuel, via Gasification or Steam Reforming. For Carbonaceous fuels that are solid, liquid, or gaseous, partial oxidation to Syngas is possible, followed by Carbon Dioxide capture. Similarly, Steam Reforming can be used to convert liquid and gaseous Hydrocarbon fuels to Hydrogen. Obviously, significant investments are required for the Air separation or Hydrogen production plants and associated utility systems. Additional operating costs for Air separation and Hydrogen production also affect the economics. A credible business case for such investments does not exist at the present time. In future, however, increased regulatory and stakeholder pressures, Carbon reduction incentives or punitive Carbon taxes might create viable opportunities for this approach.
The third approach, namely, Oxy-combustion, also involves capture of Carbon Dioxide from Flue gases, albeit with volume reduction achieved by the prior removal of Nitrogen from combustion Air. Some modifications on the combustion systems and fired equipment may be needed in existing plants, in addition to Air separation and utilities. This approach definitely improves the overall economics of capture, though the Carbon Dioxide stream has a relatively higher concentration of impurities needing eventual removal.
The capability for brownfield retrofitting with minimal disruption has driven industry preference towards Post-combustion (i.e. Flue gas) Carbon capture options. Policy makers also see this as a potential early-win option in achieving Carbon emission reduction targets. Hence such projects generally get the benefits of policy support. Given this directional thrust, the differentiators among various post combustion capture technologies are CAPEX, OPEX, efficiency, reliability, safety, and environmental aspects. Each industrial sector and project has unique features that need consideration while selecting Flue gas Carbon Dioxide capture technologies.
3 Technologies for Flue Gas Carbon Dioxide Capture
Commercial and emerging technologies for Flue gas Carbon Dioxide removal are based on the following mass transfer modes [4]:
- Absorption in solvents
- Adsorption onto solid surfaces
- Cryogenic separation
- Membrane separation
The main features of these technologies are described in the following sections.
3.1 Absorption in solvents
Solvent absorption is the most commonly used technology for Flue gas Carbon Dioxide capture. The concept of solvent absorption is based on the fact that some solvents have an affinity for Carbon Dioxide, either due to solubility or reactivity. These solvents have the ability to absorb Carbon Dioxide at higher pressures or at lower temperatures and release it when the pressure is reduced, or the solution is heated. Apart from satisfying absorption efficiency and energy demand criteria, solvents must withstand degradation during repeated absorption/desorption cycles and be non-hazardous.
Figure 3 illustrates the concept of solvent absorption. Cooled Flue gases, after contaminant removal, are contacted by lean solvent in a counter-current absorption column where typically 95% of the Carbon Dioxide is removed. Clean Flue gases exit from the top of the absorption column and rich solvent leaves at the bottom, en-route to the regeneration column. The regeneration process involves stripping-out absorbed Carbon Dioxide and the regenerated solvent is then cooled and recirculated to the absorber.
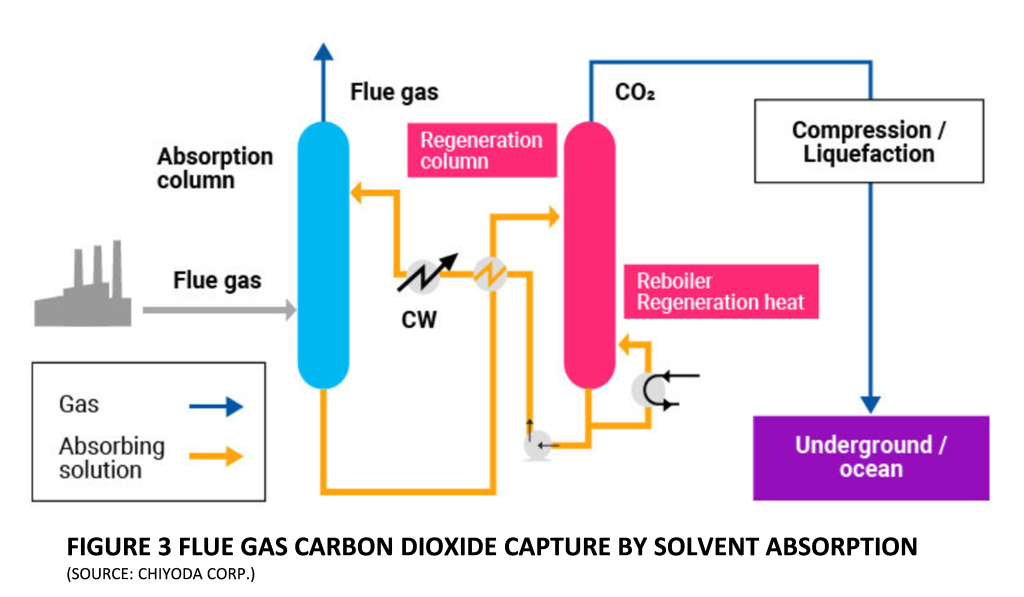
Conventionally, solvents are called physical or chemical solvents depending on whether absorption is purely based on solubility, or it involves chemical reactions. Physical absorption depends upon high partial pressure of Carbon Dioxide in the gas phase to drive the process. Flue gases on the other hand are discharged at atmospheric pressure and the partial pressure of Carbon Dioxide is relatively low. Hence the solvents used in Flue gas Carbon Dioxide removal are usually chemical solvents. There are some specialty hybrid solvents as well, which involve both physical and chemical capture mechanisms.
A wide variety of chemical solvents have been successfully used for post-combustion Carbon Dioxide capture. Most of them are formulations containing Amines. It is convenient therefore, to place commercially important solvent processes in the following categories:
- Amine Solvents
- Non-Amine Solvents
3.2 Amine Solvents
Absorption by Amine solvents has been the mainstay of Carbon Dioxide separation in the Gas Processing and Chemical Process industries for several decades. These proven technologies have therefore been a “go-to” option to reduce the investment risks associated with large scale deployment in post-combustion Carbon Dioxide capture applications.
Amines, also referred to as Alkanolamines, are a group of alkaline chemicals related to Ammonia. The replacement of one or more Hydrogen atoms in Ammonia (NH3) by an organic group results in an organic Amine.
 Primary Amines are the group of compounds where one Hydrogen atom in the Ammonia structure is substituted by one organic group, secondary Amines have two Hydrogen atoms substituted by two organic groups and tertiary Amines have all three Hydrogen molecules replaced by organic groups [5]. Figure 4 shows the molecular structures of Amines commonly used to remove acid gases like Carbon Dioxide and Hydrogen Sulphide in conventional gas sweetening processes.
Primary Amines are the group of compounds where one Hydrogen atom in the Ammonia structure is substituted by one organic group, secondary Amines have two Hydrogen atoms substituted by two organic groups and tertiary Amines have all three Hydrogen molecules replaced by organic groups [5]. Figure 4 shows the molecular structures of Amines commonly used to remove acid gases like Carbon Dioxide and Hydrogen Sulphide in conventional gas sweetening processes.
Amine solvent processes for industrial post-combustion Carbon Dioxide capture applications are mostly based on Mono-Ethanol Amine (MEA). This is due to its high reactivity with Carbon Dioxide and relatively low solvent cost. It is reasonably resistant to degradation across multiple operating cycles. Technical knowhow and operating data for aqueous MEA systems are available in the public domain. The data indicates that despite its widespread use, aqueous MEA solution is not the optimum solvent for Flue gas applications. Disadvantages include high energy requirements, formation of stable Carbamates and heat stable salts, high oxidative degradation, high vaporization losses and high corrosivity at concentrations above 20%. Several improved Amine formulations that use MEA and other Amines have been developed to mitigate these issues and tackle the wide range of Flue gas Carbon Dioxide capture applications in industry.
3.2.1 Typical Amine Absorption Process
The principle underlying all Amine Absorption Technologies are similar. Conventional aqueous MEA is customarily used as a benchmark to compare various Amine solvents. MEA solution concentrations used in Flue gas Carbon Dioxide capture range from 15 % to 30% by weight of MEA in Water. MEA undergoes a reversible and exothermic reaction with Carbon Dioxide in aqueous solution.The reaction scheme is complex, since Carbon Dioxide dissolves in Water to form Carbonic Acid, which partially ionizes to release Protons that are picked up by MEA. This step is called Protonation of Amine and it facilitates dissolved Carbon Dioxide transformation to Bicarbonate ion. This allows additional Carbon Dioxide to be absorbed. MEA also reacts directly with dissolved Carbon Dioxide to form Carbamate (RNHCOO–). During regeneration the reactions are reversed. The overall simplified scheme is as follows [8]:
Carbon Dioxide absorption: 2 R-NH2 + CO2 → R-NH3+ + R-NH-COO–
MEA Regeneration: R-NH-COO– + R-NH3+ + (Heat) → CO2 + 2 R-NH2
As can be seen, two moles of Mono-Ethanol Amine are required to remove one mole of Carbon Dioxide. This is one of the disadvantages of the conventional MEA process since it necessitates high Amine circulation rates and correspondingly high energy consumption.
Figure 5 is a typical process scheme used for the Conventional MEA Absorption process.
Hot Flue gas, after removal of impurities, and waste heat recovery, undergoes quenching in a direct-contact cooler (DCC), using a spray of deionized Water or cooled Steam condensate. Thereafter, cooled Flue gas enters the Amine absorption tower for removal of Carbon Dioxide. In the absorption tower, lean Amine solution from the regenerator flows down, establishing counter-current contact and mixing with the ascending Flue gas. The tower may be a packed or trayed column. Stainless steel materials are used due to the corrosivity of MEA and Carbonic Acid.
At any point, the difference between the partial pressure of Carbon Dioxide in the Vapor Phase and its corresponding equilibrium partial pressure establishes the driving force for mass transfer. At the top and bottom of the absorption column, Amine solvent will be at about 80% of its equilibrium concentration with Carbon Dioxide at the corresponding temperature, so that the driving force is maintained.
The path ABCD in Figure 5, shows the typical operating cycle of MEA solvent absorption and regeneration, plotted on Carbon Dioxide-MEA equilibrium curves. At point “A”, lean MEA solvent is fed to the top of the absorber and then picks up Carbon Dioxide from Flue gas, with temperature rising due to the exothermic reaction. Rich MEA leaves the absorber at “B”, typically at 60 oC, pre-heated in the cross flow heat exchanger (XFHE) and fed to the top of the stripper at “C”. The liquid flowing down the stripper packing or trays is heated further, by hot reboiler vapour passing upwards and releases Carbon Dioxide. Typically, there will still be 0.15 – 0.35 moles of Carbon Dioxide per mole of Amine in the lean MEA leaving the stripper at the bottom, point ”D” which is at around 120 oC.
To avoid continuous build-up of heat stable salts (HSS), a slip-stream of lean Amine is routed to the reclaimer. Here, Caustic Soda is used to neutralise heat stable salts and Water, Carbon Dioxide and Amine are evaporated, to leave all solid impurities in the reclaimer [6].
3.2.2 Commercially Important Amine Solvent Processes
The Amine based processes used in Commercial operation or near-commercial demonstration projects are as follows [6]:
- Kerr-Mcgee/ABB Lummus Crest MEA process
- Fluor Daniel Econamine FG process
- MHI hindered amine (KM CDR) process
- Carbon Clean Solution Ltd. (CDRMax™)
- Shell Cansolv process
- TCM CESAR1 Solvent
These are summarized in the following sections along with example projects.
3.2.2.1 Kerr -McGee/ABB Lummus Crest Process [10]
This is the earliest commercial application of aqueous MEA to post-combustion Carbon Dioxide capture. The process uses 20% by weight aqueous MEA solution and the process scheme is as described in section 3.2.1.
As mentioned earlier MEA has rapid kinetics but requires high regeneration energy compared to the newer Amine solvents. Consequently, the effective output from a power plant could reduce by more than one-third of its capacity when integrated to a Flue gas Carbon Dioxide plant using aqueous MEA solution [9].
Example Project
The Searles Valley Minerals Plant in Trona, California, is the first commercial large-scale Flue gas Carbon Dioxide capture plant in the United States. It was set up in 1978, using the Kerr-McGee/ABB Lummus Crest Process and is still in operation. It comprises two parallel trains each capturing 363 metric tons/day of Carbon Dioxide from the flue gases emitted by a Coal-fired boiler. The Carbon Dioxide is utilized is used to carbonate Brine for Soda Ash manufacture. This aspect also makes the project an early example of CCU (Carbon Capture and Utilization). It may be noted that a typical 500 MW Coal-fired power plant, generates about 12,500 tonnes Carbon Dioxide/day.
3.2.2.2 Fluor Daniel Econamine FG plus process [11]
The “ Econamine FG plus” process has been developed by Fluor Corporation based upon the well-known Dow Amine process for Acid Gas removal. Fluor purchased the license from Dow Chemical in 1989 and subsequently improved the technology for Flue gas Carbon Dioxide capture. Energy requirement for Solvent regeneration is a major operating cost and the Econamine technology reduces Steam consumption by over 30%, compared to the conventional MEA process. The solvent formulation enables Carbon Dioxide removal from low-pressure, Oxygen-containing streams without rapid oxidative degradation.
Fluor have been successful in licensing this technology to dozens of power and industrial projects worldwide, ranging from Coal and Gas Turbine power plants to Steam-Methane Reformers. Each type of application has unique technical aspects that need consideration.
For example, Gas Turbine flue gases are characterised by low Carbon Dioxide concentration while Oxygen concentration is relatively high. Further, back-pressure and pressure fluctuations affect turbine performance. The solvent must resist oxidative degradation and have good Carbon Dioxide pickup at low partial pressures. Figure 6 illustrates the typical scheme of an “Econamine FG plus” unit at a Gas Turbine based power plant.


A Coal-fired power plant, on the other hand, poses a different set of problems related to Flue gas Carbon Dioxide capture. Flue gases even after pollution control treatment, can contain Sulphur Dioxide, Ammonia (from De-NOx units), condensed Sulfuric acid, or Hydrochloric acid droplets, particulates, and other trace components. Acidic impurities react with Amine to form heat stable salts that build up in the system and affect performance. The “Econamine FG plus” process for Coal-based plants therefore includes pre-treatment to remove these impurities. This is illustrated in Figure 7, which is a typical process scheme for a Coal-based power plant. Here a selective catalytic reduction system involving Ammonia injection is used to remove Oxides of Nitrogen. Other steps shown include adsorbent injection for Sulphur-Trioxide gas and even Activated Carbon injection for adsorbing Mercury. Subsequently particulates are captured by ESP or fabric filters and a wet or dry Lime based Flue gas Desulphurization unit ensures complete removal of Sulphur Oxide impurities.
Example Project
An early and successful commercial scale application of the Econamine FG plus technology was the unit established for Florida Power and Lights Gas Turbine Power plant at Bellingham, Massachusetts, USA, in 1991. The plant was designed to capture 365 short tons per day of Carbon Dioxide from the power plant Flue gases. It was operated and maintained by Fluor from 1991 to 2005.

3.2.2.3 MHI Hindered Amine (KM-CDR™) Process
This process called the KM-CDR™ (Kansai Mitsubishi Carbon Dioxide Recovery) was developed by Mitsubishi Heavy Industries (MHI) and Kansai Electric Power Co., Inc. It uses a sterically hindered Amine solvent named KS-1™. Sterically hindered Amines have relatively bulky organic groups connected to the Amine group. They can be designed to inhibit Carbamate formation and hence ensure higher free Amine availability for Carbon Dioxide dissolution and hydrolysis, by Protonation. MHI have commercially deployed their KS-1™solvent, and further developments such as KS-2 and KS-3 have been reported. The KS-1™ solvent circulation rate is about 60% of that of the MEA process, which reduces pumping costs, as well as equipment sizes. Further, the heat of absorption is 20% lower than that of MEA, hence the regeneration energy requirement reduces by a corresponding amount [7].
It may be noted however, that the unit cost of the solvent is higher by a factor of about five when compared to MEA. The KS-1™solvent also requires low levels of SOx and NOx (typically 1ppm) and therefore requires upstream polishing of Flue gases to achieve these absorber input requirements [10].
Example Project
MHI’s reference list includes thirteen Carbon Dioxide capture plants to produce Fertilizer, Methanol and Enhanced Oil Recovery [12]. Their most significant project using KM-CDR™ has been the Petra Nova post-combustion Carbon capture project, This project is located at the W.A. Parish generating station in Fort Bend County, Texas, USA. It is designed to remove more than 90% of the Carbon Dioxide from a slipstream of Flue gas of a 240 MW Coal-fired power plant boiler The facility was commissioned in December 2016, with the captured Carbon Dioxide being utilized for Enhanced Oil Recovery at the West Ranch Oilfield [13].
Petra Nova has a Carbon Dioxide capture capacity of 1.4 million metric tons of per year. Figure 9 is a simplified Process Flow scheme of the Carbon Dioxide capture unit.

3.2.2.4 Carbon Clean Solution Ltd. (CCSL) Process [12]
The solvent used in the CCSL process is a mixture of hindered Amine (2-Amino-2-Methyl-1, 3-Propandiol) with the Potassium salts of Amino Carboxylic or Sulfonic acids and about 75 weight % of Water. This mixture is termed Amine Promoted Buffer Salt (ABPS). The Process is called CDRMax™, The CDRMax™ process improves upon the following aspects of the conventional MEA process:
- Energy consumption reduces by 27%
- Amine losses reduced by eight times
- Significantly less corrosive than MEA
Example Project
CCSL’s first commercial project with a Carbon Dioxide capture capacity of 60,000 metric tons per year was commissioned in India in 2016, at Tuticorin Alkali Chemicals and Fertilizers. The recovered Carbon Dioxide from a Coal-fired boiler is used to make Soda Ash.
3.2.2.5 Shell Cansolv Process [10]
Cansolv used to be a subsidiary of Union Carbide and was purchased by Shell Global Solutions International B.V. The Cansolv process uses an aqueous Diamine Solvent that can be tailormade to selectively remove Carbon Dioxide, Sulphur Dioxide and Oxides of Nitrogen. This makes it suitable to treat Coal-based power plant Flue gases. For example, Cansolv DC103 captures Carbon Dioxide whereas Cansolv DS removes Sulphur Dioxide. Thus, by using the two solvents sequentially, both Sulphur Dioxide and Carbon Dioxide can be removed (note: Oxides of Nitrogen are more easily removed by Selective Catalytic Reduction). The Cansolv DS Sulphur Dioxide capture system produces Sulphuric Acid as a byproduct. Heat integration in the design ensures that heat of absorption in the Sulphuric Acid unit can be harnessed to provide thermal energy elsewhere in the process.
Example project
The Cansolv process incorporating Sulphur Dioxide and Carbon Dioxide capture was implemented at SaskPower’s Boundary Dam Coal-fired power plant located in Estevan, Saskatchewan, Canada. The project was commissioned in 2014. The Carbon Dioxide capture unit was retrofitted to a 150 MW Coal-fired power unit in the complex. The process used Cansolv DS to achieve 100% Sulphur Dioxide capture before routing the Flue gases to a Cansolv DC103 Carbon Dioxide capture system. After retrofitting, the 150 MW unit could produce 115 MW net power for sale. The Steam turbine and generator were replaced in the retrofit. The Carbon capture capacity of the Cansolv plant is one million tonnes per annum of Carbon Dioxide, which is sent for sequestration. This is in fact the first Carbon Capture and Sequestration project to have been implemented on a Coal-fired Power plant [10,14].
3.2.2.6 TCM’s CESAR1 Solvent
The CESAR1 solvent is at an advanced testing stage, operating at near-commercial scale. It has been developed at the Technology Centre Mongstad (TCM DA), located next to Equinor refinery in Norway. TCM is a joint venture between Gassnova, Equinor, Shell and Total Energies.
Due to its proximity to the refinery, the solvent has been tested on Flue gases from the refinery’s Combined Cycle Gas Turbine power plant (CCGT) and Residue Fluid Cracker Unit (RFCCU), as well as other refinery units such as Steam Methane Reformer (SMR). The CESAR1 solvent is a blend of 27 wt.% 2-Amino-2- methylpropanol (AMP) and 13 wt.% piperazine (PZ) and is considered to be a better solvent than MEA in terms of thermal energy and stability [17].

Figure 10 is simplified process flow diagram of the TCM CESAR1 plant. Flue gas from the blower is cooled to the required temperature (normally 20-50°C) in the Direct Contact Cooler (DCC). The lean Amine solvent can be fed either at 12m, 18m or 24m to the packed bed absorber (highlighted in yellow in Figure 10). Rich Amine from the absorber is pumped through the rich/lean cross plate heat exchanger to the top of CHP stripper. A slip-stream of the cold rich Amine is bypassed upstream the heat exchanger to the stripper overhead.
Example project
TCM has conducted several test campaigns using CCGT and RFCCU flue gases. The main objective of these campaigns has been to benchmark the performance of the CESAR1 solvent in terms of energy demand, emissions and solvent consumption. The CCGT tests have captured 80 metric tons/day of Carbon Dioxide while the RFCCU gases capture rate has been 200 metric tons/day. There are many operational challenges that need to be overcome before commercialization. It has been verified that the energy demand is lower by around 10% compared to 30 wt.% MEA.
3.3 Non-Amine Solvents
In contrast to the variety of Amine-based Flue gas Carbon Dioxide capture technologies, there is only one non-Amine option of commercial significance at this time. This is Alstom’s Chilled Ammonia Process. Other non-Amine Solvents do exist in the Gas Treatment industry but have not found favour for Flue gas Carbon Dioxide capture applications. Newer options such as ionic liquids are at development stage.
3.3.1 Alstom Chilled Ammonia Process
Alstom’s patented Chilled Ammonia Process (ACAP) addresses the problems faced by Amine Solvents such as oxidative degradation, formation of heat stable salts, reactions with Oxides of Sulphur and Nitrogen and evaporative losses. The ACAP process uses the chemical equilibrium characteristics of a mixed Carbon Dioxide, Water, Ammonia, Ammonium Carbonate, and Ammonium Bicarbonate solution at various temperatures, to absorb and release Carbon Dioxide. It also allows the solvent regeneration to be performed at higher temperature than allowed for MEA. This means that the regenerator can operate at a higher pressure and Carbon Dioxide can be released at higher pressure to downstream processes for utilization or sequestration. The ACAP Process requires a larger absorber than in the case of MEA, because it has a relatively low reaction rate and Carbon Dioxide loading, and loses some efficiency to the Chiller, which is required to maintain reaction temperature. There are also scaling issues that have been reported. Figure 11 illustrates the process scheme of the ACAP Process.

The ACAP scheme involves two-stage cooling and chilling of Flue gases to about 35 0F (1.7 0C), by direct contact with refrigerated Water. Condensed Water drops out of the chilled Flue gas. The cooling circuit consumes about 1% to 2% of the power plant’s power output, but significantly reduces the compression power requirements thereafter. Subsequently, Carbon Dioxide is removed in the absorber by contacting with lean solvent. The rich solvent containing absorbed Carbon Dioxide is then pumped for regeneration at elevated pressure and temperature [10,15].
Example project [16]
In 2009, Alstom Power commissioned a chilled-Ammonia CCS technology validation project at American Electric Powers Mountaineer Plant in New Haven, WV, USA. AEP’s Mountaineer plant is a 1,300-megawatt electrical (MWe) coal-fired unit that was retrofitted in 2009 with Alstom’s patented chilled ammonia CO2 capture technology on a 20 MWe portion, or slipstream, of the plant’s exhaust Flue gas. The Flue gas is taken downstream of the plant’s existing Selective Catalytic Reduction (SCR), particulate control, and wet Flue gas desulfurization (WFGD) systems. The slipstream of Flue gas is chilled and contacted with a solution of Ammonium Carbonate, which absorbs the Carbon Dioxide to create Ammonium Bicarbonate. The Ammonium Bicarbonate solution is then regenerated by heating under pressure to produce a high-purity stream of Carbon Dioxide. The Carbon Dioxide is sent for sequestration.
3.4 Limitations of Solvent Absorption Technologies
Solvent Absorption processes for post-combustion capture of Carbon Dioxide have to handle large volumes of hot, contaminated, low-pressure Flue gases. This inevitably leads to the following disadvantages:
- Large equipment sizes for Absorbers, Regenerators, Heat exchangers. Compressors and Pumps, increasing the CAPEX.
- High requirements of electrical power and other utilities. In the case of power projects, the parasitic load results in considerable derating of power plant capacity.
- Solvent degradation due to reactions with impurities in the flue gases.
- Handling large volumes of corrosive and toxic solvents
- Unavoidable emissions of solvent vapours, and disposal of spent solvents creating environmental problems
3.5 Adsorption onto Solid Surfaces
Adsorption processes using solid regenerable sorbents capable of capturing Carbon Dioxide from Flue gas streams can potentially overcome many of the disadvantages of Solvent processes. These include reduced energy for regeneration, greater Carbon Dioxide removal capacity, selectivity, and ease of handling. Potential disadvantages for adsorbents include particle attrition, handling of large inventories of adsorbents and thermal management of adsorber vessels [10].
3.5.1 Types of Adsorption Processes
Adsorption processed are grouped into physical adsorption, due to weak Van der Waals forces or chemisorption ,where stronger covalent bonds are established between Carbon Dioxide molecules and binding sites on the adsorbent surface. Both physical and chemical adsorbents are used for Flue gas Carbon Dioxide capture. Suitable adsorbents must have selectivity for Carbon Dioxide, sufficient adsorption capacity, enable adequate kinetics of adsorption mechanical strength to withstand pressure and temperature cycling. Adsorption systems may use packed-bed or fluidized-bed configurations [10].
Figure 12 illustrates the principles of adsorption processes for Flue gas Carbon Dioxide capture.

Adsorption processes have not yet been deployed at commercial scale. Various adsorbent materials that are being researched include Zeolite 13X, Metal Organic Framework (MOF), Activated Carbon, supported Alkali Metal Carbonates, Metal Oxides and Alkali Metal salts.
3.6 Cryogenic Separation [5]
Cryogenic separation of Carbon Dioxide from Flue gases is at early research and development stage. This technology is more likely to be applied for pre-combustion capture of Carbon Dioxide from Fuel gas. This is because a lot of experience has been gained with low temperature separation of Carbon Dioxide from Natural Gas and Syngas in the Hydrocarbon Industry.
The underlying principle is to separate Carbon Dioxide as liquid at high pressures or as solid by freezing. The cooling can be accomplished by Joule Thomson expansion of the process gas or by external refrigeration, and the liquid temperature is typically below -60 0C.When cooled Below -78 0C Carbon Dioxide will freeze.
At least two cryogenic technologies have been publicly demonstrated at near-commercial scale for Natural gas applications, as follows:
- Cool Energy’s CryoCell® demonstration plant for Carbon Dioxide separation from Natural Gas, located in the Perth basin, Western Australia. The CryoCell® process was developed by Cool Energy Ltd and tested in collaboration with other industrial partners including Shell Global Solutions.
- Exxon Mobil’s Controlled Freeze Zone technology This is a single-step cryogenic separation process that removes Carbon Dioxide from Natural Gas in a specially-designed section of a distillation tower, where Carbon Dioxide is allowed to freeze and separate out in a controlled manner. It can then be melted and distilled to further purify it. A commercial scale demonstration plant to remove Acid gases from Natural Gas was operated from at Shute Creek Treatment Facility at LaBarge, Wyoming, USA, from March 2012 to November 2013 .
3.6 Membrane Separation[19]
Membranes are currently used commercially for Carbon Dioxide removal from Natural Gas at high pressures. However, there is only one technology that is currently available at pre-commercial stage, for Flue gas Carbon Dioxide capture. This is the process developed by Membrane Technology and Research, Inc., (MTR) USA. Their membrane-based post-combustion Carbon Dioxide capture technology uses a new class of membranes, called Polaris™, that has ten times the Carbon Dioxide permeance of conventional gas separation membranes. A tenfold increase in permeation rates leads to correspondingly smaller membrane units, decreasing the CAPEX.
Example project
MTR has operated a small pilot system using the Polaris™ membrane system in slipstream tests at the National Carbon Capture Centre (NCCC). The system captured 20 metric tons per day of carbon Dioxide .Now it is proposed to install a large pilot to achieve about 70% Carbon Dioxide capture from a 10 megawatt electricity (MWe) equivalent slipstream of Flue gas. This works out to 140 metric tonnes per day of Carbon Dioxide. The proposed process scheme and skid layout are shown in Figure 13.

MTR Process Description for Large Pilot
Flue gas to the unit will be at 85°C, having about 12.7% Carbon Dioxide and 18% Water. A blower boosts the Flue gas pressure to about 1.2 barA. Thereafter, the Flue gas is quenched in a Direct Contact Cooler (DCC), which reduces the Water content of the gas to 1.5% and increases the Carbon Dioxide content to about 15%. The gas subsequently enters the first-stage membrane modules, where it splits into a Carbon Dioxide enriched permeate (about 50% Carbon Dioxide) and a vent gas (about 4% Carbon dioxide), which is routed to the vent stack. A vacuum pump maintains about 0.1 barA pressure on the permeate-side of the membrane and ensures sufficient permeate flow rate. The vacuum pump discharges at about 1.1 barA, with the first stage permeate going to a second-stage membrane, which further enriches the gas to about 85% Carbon Dioxide. The second-stage membrane unit also uses a vacuum pump on the permeate side. The permeate gas from the second membrane stage is further boosted to 25 barA by a compressor. Some dehydration occurs due to compression and cooling to about 5 0C. A Molecular Sieve drier then produces dry Carbon Dioxide gas. The dry gas is further subjected to cryogenic distillation to get high purity liquid Carbon Dioxide.
4 Conclusion
The following aspects of Flue gas Carbon Dioxide capture technologies are apparent from this review:
- Removal of Carbon Dioxide from Flue gases is more challenging than traditional Natural Gas sweetening or similar well-established processes in the Fertilizer and Hydrocarbon Industry. Low-pressures, large volumes and presence of Nitrogen, Oxygen and impurities are some of the challenges.
- Flue gas Carbon Dioxide capture is currently preferred to other approaches like Pre-combustion or Oxy-combustion. This is due to lesser CAPEX as well as retrofit capability that minimizes disruption to existing operations.
- Coal and Gas turbine power plants have seen the maximum focus and deployment of Flue gas Carbon Dioxide capture technologies.
- Solvent Absorption is the only technology that is established at a commercial scale for Flue gas Carbon Dioxide capture. Amine-based solvents completely dominate the market at present.
- While MEA is the oldest Amine solvent in use, several proprietary Amine-based solvent have been commercially deployed with much better performance than MEA. They may however be costlier.
- Membrane and adsorption technologies are expected to improve the operability of Flue gas Carbon Dioxide capture plants since they are dry processes. Energy consumption would be reduced, and safety and environmental issues related to solvent handling would be eliminated.
5 References
- Global Energy Review: CO2 emissions in 2021; Flagship Report, March 2022, IEA; https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2
- Global Greenhouse Gas Emissions Data: United States EPA; https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data
- “CO₂ and Greenhouse Gas Emissions”; by Hannah Ritchie, Max Roser and Pablo Rosado (2020) – Published online at OurWorldInData.org. https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions
- Report of the Mission Innovation Carbon Capture, Utilization and Storage Experts’ Workshop Hilton Hotel North, Houston Texas September 25–29, 2017. https://www.energy.gov/fe/downloads/accelerating-breakthrough-innovation-carbon-capture-utilization-and-storage
- Assessing the Cost Reduction Potential and Competitiveness of Novel (Next Generation) UK Carbon Capture Technology Literature Review, Document Number: 13333-8820-RP-003 Date: 21st February 2018 Revision: 2A
- BAT Review for New-Build and Retrofit Post-Combustion Carbon Dioxide Capture Using Amine-Based Technologies for Power and CHP Plants Fuelled by Gas and Biomass as an Emerging Technology under the IED for the UK, UKCCSRC Report, Ver.1.0, July 2021. By Gibbins, J., Lucquiaud, M. (2021).
- Flue gas treatment for CO2 capture; by Deborah Adams IEA Clean Coal Centre, June 2010.
- IECM Technical Documentation: Amine-based Post-Combustion CO2 Capture; https://www.uwyo.edu/iecm/
- Current status and future development of solvent-based carbon capture; by Eni Oko, Meihong Wang & Atuman S. Joel; Int J Coal Sci Technol (2017) 4(1):5–14. https://link.springer.com/article/10.1007/s40789-017-0159-0
- Post Combustion Capture (PCC) January 2012, Global Carbon Capture and Storage Institute, Canberra, Australia. https://www.globalccsinstitute.com/archive/hub/publications/29721/co2-capture-technologies-pcc.pdf
- 11.Commercially Available CO2 capture technologies, POWER, Aug 1, 2009. https://www.powermag.com/commercially-available-co2-capture-technology/
- A critical analysis of CO2 capture technologies; by Jha, A., Ravuru, V., Yadav, M., Mandal, S., Das, A. K.; Hydrocarbon Processing, June 2021. https://www.hydrocarbonprocessing.com/magazine/2021/june-2021/sustainability/a-critical-analysis-of-cosub2sub-capture-technologies
- World’s Largest Post-Combustion Carbon Capture Project Completed, POWER, Jan 10, 2017. https://www.powermag.com/worlds-largest-post-combustion-carbon-capture-project-completed/
- What are the top carbon capture and storage projects around the world?; NS Energy,19 July,2019; https://www.nsenergybusiness.com/features/top-carbon-capture-storage-projects/
- Alstom’s chilled ammonia CO2-capture process advances toward commercialization; POWER, Feb 15, 2008. https://www.powermag.com/alstoms-chilled-ammonia-co2-capture-process-advances-toward-commercialization/
- Alstom Announces Successful Results of Mountaineer Carbon Capture and Sequestration (CCS) Project; Alstom Press Release, 5 May 2011. https://www.alstom.com/press-releases-news/2011/5/alstom-announces-sucessful-results-of-mountaineer-carbon-capture-and-sequestration-ccs-project
- CO2 Capture from SMR type flue gas using CESAR1 solvent at Technology Centre Mongstad; by Sundus Akhter , Ahmad Wakaa , Anette Knarvik, Erik Gjernes, Ida M. Bernhardsen, Muhammad I. Shah, 16th International Conference on Greenhouse Gas Control Technologies, GHGT-16 23rd -27th October 2022, Lyon, France
- First Process Results and Operational Experience with CESAR1 Solvent at TCM with High Capture Rates (ALIGN-CCUS Project); by Christophe Benqueta, Anette Knarvik, Erik Gjernes, Odd Arne Hvidsten, Eirik Romslo Kleppe, Sundus Akhter; 15th International Conference on Greenhouse Gas Control Technologies, GHGT-15, 15th– 18th March 2021 Abu Dhabi, UAE.
- Large Pilot Testing of the MTR Membrane Post-Combustion CO2 Capture Process; U.S. Department of Energy, National Energy Technology Laboratory, Project No. FE0031587, 2018.

 To all knowledge
To all knowledge