In this article, EPCM reviews the Process of Helium Recovery from Natural Gas and conventional processes for recovering helium from natural gas. It examines the potential of emerging technologies for more efficient helium production processes, with the main focus on Southern Africa’s new helium-rich gas reserves.
Helium is a valuable noble gas with unique properties used in various applications, including the medical, nuclear and space industries (Soleimany, Hosseini & Gallucci, 2017). Despite its status as the second most abundant element in the universe, the only commercially viable source for helium recovery is natural gas reserves (Sunarso et al., 2016). Helium extraction from gas sources containing more than 0.05 % (vol) helium has been proven economical (Kim, 2014).
Separating helium from natural gas aims to increase natural gas’s heating values and helium recovery for higher returns (Mehrpooya & Shafaei, 2016). In 2010, the global demand for helium reached 30,000 tons, amounting to US$1 billion (Soleimany et al., 2017). The demand in the next two decades is expected to double (5-7 % increase per annum) (Sunarso et al., 2016). Helium extraction facilities currently in operation are listed in Table 1.
As can be seen, the United States accounts for nearly 71% of global helium production plants, followed by Algeria, Qatar, Poland and Russia. Currently, 21 nuclear power plants (mostly in Asia) with 150 reactors are under construction, which will also require enormous amounts of helium for cooling systems in the near future (Rufford et al., 2014). It is clear that current production plant capacities fail to meet the projected demands, leading to constant yearly increases in helium prices (illustrated in Figure 1).
Recently, two valuable helium-rich natural gas resources have been discovered in Southern Africa. South Africa’s Virginia gas field contains up to 4 % helium, and Tanzania’s Craton basin in Rukwa province shows samples that contain up to 10 % helium. Table 2 provides a range of compositions of helium-rich gas from various global natural gas fields.
Table 1: List of Global Helium Extraction Plants (West, 2009)
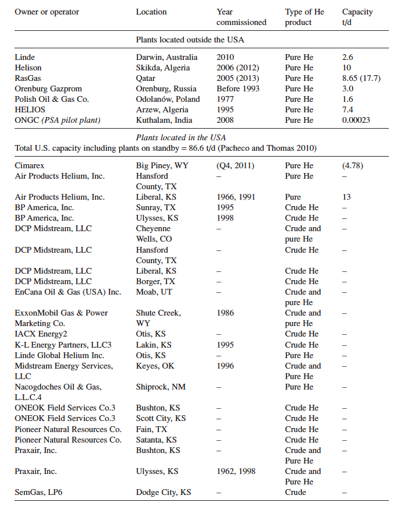

Figure 1: Helium price comparison with other natural resources (USGS, World Bank)
Table 2: Composition of helium-rich natural gas fields (Häussinger et al., 2005)

Indicated in the high helium compositions recently discovered in Southern African gas reserves compared with other helium “rich” resources. This highlights Africa’s untapped potential, waiting to be unlocked, even before considering neighbouring countries’ rapidly growing natural gas discoveries.
This article reviews conventional processes for recovering helium from natural gas. It examines the potential of emerging technologies for more efficient helium production processes, with a main focus on Southern Africa’s new helium-rich gas reserves.
1 Process of Helium Recovery from Natural Gas Review: Helium separation and recovery technologies
1.1 Overview of helium extraction from natural gas
When performing helium extraction from natural gas, the helium is first separated/recovered from the bulk fluid, where further purification is done. Figure 2 is a block-flow illustration of a general process for helium recovery from natural gas (Soleimany et al., 2017).

Figure 2: Schematic representation of a typical helium recovery process from natural gas (Soleimany et al., 2017)
Natural gas compositions vary depending on resource locations. Common natural gas resources consist of 30-90 % methane and light hydrocarbons. In addition, other gases that form along with natural gas are nitrogen, hydrogen sulphide, water, carbon dioxide, and traces of heavy metals (Hosseini, 2009; Hosseini & Najari, 2016).
Tanzania’s Itumbula, Rukwa Spring’s deep crustal gas compositions illustrated 8-10.2 % helium with approximately 90 % nitrogen (Helium One, 2017). The Virginia microbial gas field in South Africa has a proven 4 % and probable 10 % helium-containing reserve, with the remaining gases being methane and mostly small amounts of water vapours.
To extract helium, all other impurities and contaminants must be removed following industry standards. The process of extracting and producing liquefied helium from natural gas consists of six steps (Soleimany et al., 2017):
- Natural gas processing/pretreatment (removal of hydrogen sulphide, carbon dioxide, water and heavy metals);
- Natural gas refrigeration (removal of heavier hydrocarbons, if any) and liquefaction (production of liquefied natural gas);
- Nitrogen rejection (removal of nitrogen) / helium recovery from natural gas;
- Helium upgrading;
- Helium purification; and
- Helium liquefaction.
The pretreatment process is imperative for removing acid gases, water and heavy metals (commonly mercury) before entering the refrigeration and liquefaction process. Helium has an extremely low boiling point (seen in Table 3). Therefore, any helium in the natural gas feed to the LNG production plant is concentrated in the overhead product of a nitrogen rejection unit (NRU). This process is conventionally a cryogenic distillation process where the helium recovery is integrated with the NRU. It is desirable to have a helium recovery unit; otherwise, the remaining helium is vented to the atmosphere along with nitrogen (Rufford et al., 2014).
Table 3: Physical properties of helium and other gas components encountered in helium recovery (Rufford et al., 2014)

Gas separation processes are divided into three categories: cryogenic processes, pressure swing adsorption (PSA) and membrane separation (Bakhsh et al., 2007; Crawford, Coyle & Anantharaman, 2010).
In cryogenic technologies, separation is achieved at temperatures below -65 ºC. Cryogenic separations can accomplish up to 90% helium recovery. Cryogenic processes are divided into two groups: multi-flash cycles and high-pressure distillation column processes (Victory, Miles & Oelfke, 2009).
PSA methods are based on the adsorption of gases on solid surfaces and operate at near-ambient temperatures (Rufford et al., 2014). These processes are mainly used in the pretreatment, nitrogen rejection, upgrading and purification steps of helium recovery from natural gas (to be discussed).
Membrane technologies effectively separate gas mixtures using synthetic membranes made from various materials based on the theory of Fick’s Law. They have not yet advanced, like cryogenics and PSA separation methods. However, extensive research is currently being undertaken due to the potential economic incentive membranes may have over cryogenic distillation and PSA processes (Sunarso et al., 2016).
1.2 Process of Helium Recovery from Natural Gas Review: Helium recovery by cryogenic fractionation
Cryogenics are employed in LNG production plants, where helium is extracted from the NRU after the feed gas is liquefied. While cryogenic methods for LNG production are extensively covered in other literature, this discussion will focus on the key design features of NRUs utilizing cryogenic techniques.
The four fundamental cryogenic processes used in NRUs are multi-stage flash separators, single-column heat-pumped processes, double-column processes, and dual-column cycles (Agrawal et al., 2003). The choice of process depends on factors such as the feed gas flow rate, its composition, and the helium concentration, all of which must align to ensure economically viable helium recovery. Multi-flash separator processes have higher energy requirements compared to cryogenic distillation column processes but have lower capital costs and production overhead vapour streams with low helium concentrations. Depending on the feed gas composition, a single-stage flash separator process produces nitrogen-rich overhead vapours containing 1-3 % helium. 50-70 % helium can be achieved from more complex, double-column NRU processes when operating in partial condensation mode, where nitrogen and small amounts of methane, hydrogen, neon, argon and carbon dioxides are also present (Agrawal et al., 2003; West, 2009).
Figure 3 illustrates a multi-stage flash process. The dissolved nitrogen and helium are removed from the LNG feed by reducing the pressure over a series of flash vessels.
In each flash stage, the helium is vapourised with the nitrogen, and liquid natural gas is used to pre-heat the feed. This process can also be applied to the overhead product of the NRU after it is cooled (partially condensed) to separate the gases (West, 2009). Crude helium concentrations in the products depend on the feed composition, pressure drops and temperature changes.

Figure 3: Schematic of a multi-flash process for recovering helium from natural gas (Rufford et al., 2014).
In the single-column heat-pumped process (Figure 4), a purified NG feed is pre-cooled in the main cryogenic heat exchanger (MCHE; against the rejected nitrogen) and then fed to a high-pressure column (13 – 28 bar). The vapour ejected from the top of the column contains nitrogen and helium. A closed-loop (methane used as working fluid) supplies heat to the reboiler and cooling duty to the condenser. The methane is condensed and drawn from the bottom of the tower, whereafter it is flashed over a control valve (reduction in pressure). The feed gas and rejected nitrogen from the top of the column are used to heat the methane before being compressed into downstream facilities. This process produces a nitrogen-rich stream containing approximately 1-3 % helium, depending on feed gas conditions (Rufford et al., 2014).

Figure 4: Single-column heat-pumped process (Agrawal et al., 2003)
Häussinger et al. (2005) describe a simplified process flow scheme of a modern, double-column process for nitrogen rejection and helium recovery from natural gas, as seen in Figure 5.
The feed gas is cooled against cold product streams in a cryogenic heat exchanger (main feed/product HX) and is fed to the bottom of the high-pressure column. In this high-pressure column, the helium is recovered from the feed at typical operating pressures between 10 – 25 bar. Reflux for the low-pressure and the high-pressure column is provided by the column’s partial condenser (not illustrated). The helium is contained in the non-condensed part of the high-pressure column’s overhead vapour, which is then directed to subsequent stages for further processing and purification.

Figure 5: Double-column process for nitrogen rejection and helium recovery (Häussinger et al., 2005)
The final separation of nitrogen from methane at the bottoms of the high-pressure column is carried out in the low-pressure column. The nitrogen-rich overhead product of the low-pressure column is heated in a heat exchanger against the bottoms of the high-pressure column. A reboiler is required at the bottom of the low-pressure column to achieve a low nitrogen concentration in the methane-rich residual product. The reboiler duty is provided by condensing the top nitrogen stream from the high-pressure column. The required heat transfer is possible because of the higher nitrogen stream temperature relative to the methane temperature from the bottom of the low-pressure column. A methane-rich stream is produced at the bottom of the low-pressure column, pumped to elevated pressure, vapourised, and heated against the natural gas feed (Rufford et al., 2014).
Modern cryogenic helium recovery processes are significantly more complicated than the simplified single-column and double-column systems described. The dual-column cycle shares many common features with the double-column process but has a higher level of integration between process and refrigeration streams. Depending on the local feed gas composition and available product markets, the complete, integrated cryogenic process may include the recovery of heavier hydrocarbons, fuel gas and nitrogen fractions, in addition to the recovery of crude helium (Rufford et al., 2014).
1.3 Helium upgrading and purification processes
The crude helium from the NRU must be upgraded to a helium concentration of at least 90 % before liquefaction. The impurities still contained in the crude helium, like nitrogen, methane, hydrogen and sometimes neon, must be removed in several stages. The upgrading process includes condensation of the bulk components, catalytic oxidation of any trace hydrogen, separation of water, carbon dioxide and oxygen from the reactor by condensation of the water and then within a PSA unit, and removal of the final traces of nitrogen in another PSA unit. The product from these steps can approach helium purities of 99.995 % (Daly, 2005).
Figure 6 is a schematic representation of a typical process to purify upgraded helium. The upgraded helium is mixed with air (provision of combustion oxygen), heated to above 300K and compressed through a catalyst bed to oxidise traces of hydrogen or remaining hydrocarbons. The product from the reactor is cooled to condense any water formed and sent through a water separator, from where the top gases are fed to a PSA unit. Molecular sieves in the PSA unit may be used for further dehydration, carbon dioxide and oxygen capture (Rufford et al., 2014).

Figure 6: Schematic of the upgraded helium purification process (Agrawal et al., 2003)
To attain a helium purity of 99.995 %, the hydrogen-free gas needs to flow through a low-temperature PSA unit (Lindemann et al., 2010) and/or additional cryogenic condensation processes to remove nitrogen to less than 10ppmv (Agrawal et al., 2003). Four-bed PSA molecular sieve units containing an adsorbent such as zeolite 4A are commonly used. The nitrogen blowdown gas from the PSA purification unit is compressed, dried and recycled to the inlet of the upgrader, where it is combined with the crude helium feed (Rufford et al., 2014).
Common industry processes for liquefying helium are based on isenthalpic throttling of the purified helium across a Joule-Thomson valve. The purified helium is compressed (to 20 bar) and pre-cooled to 80K with exhaust from the helium expander or liquid nitrogen, then cooled to 20K with hydrogen refrigerant or below 80K again with exhaust from the helium expander (Agrawal et al., 2003; Häussinger et al., 2005). The free expansion of the compressed gas produces the final liquid helium.
A case study is presented by (Lindemann et al., 2010) on a recently commissioned (2010) helium production facility in Australia based on direct helium separation from natural gas using cryogenic separation. The helium plant at the Darwin LNG production facility comprises a 3 % crude helium feed from an NRU (downstream of the main cryogenic heat exchangers). The plant can produce 2.6 tons per day of liquid helium (860 litres per hour) with a purity of 99.999 %. This process utilises a two-stage cryogenic flash process, a hydrogen oxidation reactor and two PSA units.
The nitrogen-rich feed is compressed to 2 bar and upgraded by cooling to 80.5 K in the first nitrogen removal stage, where part of the nitrogen is condensed to give a helium-enriched stream of 26 %. The gas is then warmed to ambient temperature, compressed to 31 bar and mixed with air to feed the hydrogen oxidation reactor. The water and carbon dioxide formed are removed in a PSA unit. After hydrogen removal, the helium-enriched gas is cooled to 81 K (second nitrogen condensation stage), producing a 93 % helium vapour stream. The upgraded helium stream is further cooled to 68 K and then flashed to provide a product of 99 % helium. Traces of nitrogen are removed (< 5 ppmv) in the final purification stage using a cryogenic PSA unit.
The world’s largest helium production facility (Ras Laffan Helium Plant in Qatar), with a production capacity of 17.7 tons per day and feed gas composition of 0.04 % helium, is based on the same cryogenic distillation principles. Each of the seven LNG production trains has its own helium recovery unit installed at the cold end of the LNG process to upgrade the crude helium produced (Daly, 2005).
1.4 Adsorption-based processes for helium recovery
Adsorption-based processes are mainly used during the pre-treatment process to remove water, carbon dioxide, hydrogen sulfide and other impurities from the feed natural gas. This process is also used to remove trace impurities of nitrogen and methane during helium purification (Tagliabue et al., 2009). Gas separation using adsorption comprises two steps: adsorption and desorption.
During adsorption, a porous solid bed selectively adsorb the higher affinity gas to the adsorbent bed and produces a gas stream enriched with the less strongly adsorbed gas component. Once the solid is saturated, the adsorbent must be regenerated. In the desorption column, the gaseous product is enriched with the strongly adsorbed component (Rufford et al., 2014).
The technologies that deploy this method are temperature-swing adsorption (TSA), PSA, fluidised adsorbent beds and moving adsorbent beds. TSA methods regenerate the gas using external heat to increase the temperature in the desorber, whereas during PSA methods, regeneration is conducted under low temperatures but by releasing pressure and purging the bed (Tagliabue et al., 2009). Fluidised and moving bed operations are less common for industrial gas separations than the cyclic-batch fixed bed operations of TSA and PSA (Saeder & Henley, 2006).
From Table 3 it is seen that the molecular size of helium (2.60 Å) and hydrogen (2.89 Å) are similar with both having very low boiling temperatures. This indicates that PSA processes used for hydrogen purification potentially have many features similar to helium purification processes. Rufford et al. (2014) stipulate the potential of new technologies under development for hydrogen purification that can also apply to helium purification. In general, modern PSA units for hydrogen purification use layered adsorbent beds with three to four adsorbents (silica, alumina for water, activated carbon for carbon dioxide, and 5A zeolite for methane and nitrogen; Ritter & Ebner, 2007). Similar multi-layer bed designs are researched for Helium recovery processes (Baksh, 2010).
It is important to note that PSA units are designed to treat a feed stream that is already upgraded (helium > 90 %); otherwise, the adsorbent will be too readily saturated by non-helium components. PSA is only used during upgrading and purification stages where nitrogen and traces of methane are removed from the helium (downstream of NRUs) (Daly, 2005; Lindemann et al., 2010).
Commercially available zeolites and narrow pore-activated carbons with reasonable nitrogen adsorption capacities make suitable adsorbents for helium purification. Table 4 lists commercial adsorbents used in industrial hydrogen purification processes that can potentially be used in PSA processes to separate heavier components from helium. Baksh (2010) also patented the use of calcium and lithium-exchanged 13X adsorbents.

Table 4: Commercial adsorbents with potential for PSA units for helium upgrading and purification (Rufford et al., 2014)
Rufford et al. (2014) discuss two case studies of direct helium recovery from natural gas using adsorption-based processes. The first is India’s Oil and Natural Gas Corporation PSA Pilot Plant, with a production capacity of 23 kilograms per day, having a feed gas containing 0.06 % helium (88.5 % methane, 9.86 % heavier hydrocarbons, 1.18 % nitrogen and 0.4 % carbon dioxide). This process has four stages: (1) pretreatment of the feed gas using PSA, (2) recovery of methane gas onto an AC adsorption bed, (3) upgrade of helium from nitrogen on a zeolite 13X adsorption bed, and (4) helium purification also using zeolite 13X adsorption bed. Although this plant illustrates the possibility of using PSA methods throughout the process, the plant only recovers 65 % of the helium in the feed gas. Conventional cryogenic processes can easily recover more than 95 % helium from the feed gas.
The second case study discussed is a US patent (no. 5542966) where a natural feed gas containing 4 % helium, 26 % hydrocarbons and 70 % nitrogen flows through a two-stage activated carbon PSA process. The production capacity is 550 kilograms per day, and a recovery of 95 % helium is reported. Although the helium recovery of 95 % is approaching cryogenic distillation process results, the nitrogen in the feed gas is high compared with conventional natural gas fields (< 10 %, 70-90 % methane). To apply to Southern Africa’s gas reserves, this process must be tested with a higher methane composition feed gas (> 80 % hydrocarbons).
1.5 Process of Helium Recovery from Natural Gas Review: Membrane separation processes for helium recovery
Gas separations performed through membrane methods are the permeation of gases through a homogeneous membrane. This is a solution-diffusion phenomenon where the ability of a membrane to separate components of a gas mixture depends on the selectivity or separation factor of gaseous components. This, in turn, is a function of the gas solubility and diffusivity coefficients (Häussinger et al., 2005). Because of the small molecular diameter of helium compared with other natural gas components, its diffusivity and, therefore, permeability in most membranes are greater, allowing for the separation of helium to be possible.
A range of membrane processes has been designed in patents, and research has been developed to recover helium from natural gas for the past 40 years (Stern et al., 1965; Scholes & Ghosh, 2017). For recovering helium directly from natural gas, membranes have demonstrated that this separation is possible when combined as two or three stages in series with recycle streams (Figure 7).
Multi-stage systems exhibit large pressure drops of the helium-rich permeate across membrane units, requiring inter-stage compressors. The recompression between membrane stages increases membrane systems’ capital and operating costs. However, these designs can enable natural gas containing as low as 1 % helium to be purified to a very high concentration while utilising existing membranes with high helium/methane selectivities (Scholes & Ghosh, 2017).
No data on the performance of helium recovery plants from natural gas utilising membrane technology has been published in the open literature, though there are various processes in patented literature (Rufford et al., 2014; Scholes & Ghosh, 2017).

Figure 7: Two- and three-stage membrane process illustrations (Scholes & Ghosh, 2017)
Two- and three-stage membrane stages in series, with recycles, have been reported as feasible for recovering and purifying helium from the overhead gas of NRUs (Scholes & Ghosh, 2016). This is due to the high nitrogen concentration and reasonable compression ratios for helium/nitrogen selectivities above 20.
Alders, Winterhalder & Wessling (2017) conducted a techno-economic comparison between various membrane-based processes for helium recovery and enrichment. Two scenarios were considered: (1) helium and nitrogen were recovered after cryogenic distillation of the natural gas, and (2) direct separation of helium from natural gas. Scenario (1) investigated the separation of nitrogen and helium by comparing (i) a pressure relief distillation, (ii) a two-stage membrane process and (iii) a second low-temperature distillation. Scenario (2) investigated direct helium removal from natural gas by comparing (i) multi-stage pressure relief, (ii) a two-stage membrane process and (iii) a three-step membrane process.
Figure 8 (pressure-relief distillation after cryogenic distillation), Figure 9 (two-stage membrane process after cryogenic distillation) and Figure 10 (two-stage distillation system) schematically illustrate the processes for Scenario (1).

Figure 8: Combined distillation and pressure relief process (Alders et al., 2017)

Figure 9: Hybrid process integrating distillation and membrane gas separation (Alders et al., 2017)

Figure 10: Two-stage low-temperature distillation system (Alders et al., 2017)
The evaluation confirmed that the hybrid process integrating the distillation and membrane technologies resulted in the lowest treatment costs, was most favourable concerning operating costs for short amortisation periods, and showed the highest helium recovery (94.2 %). All three processes were based on the same natural gas feed conditions (500 kmol/h, 80 % methane,19 % nitrogen and 1 % helium).
Figures 11 (multi-stage pressure relief), 12 (two-stage pressure relief) and 13 (three-step membrane process) show processes investigated for Scenario (2).

Figure 11: Multi-stage pressure relief system (Alders et al., 2017)

Figure 12: Two-stage membrane process (Alders et al., 2017)

Figure 13: Three-stage membrane process (Alders et al., 2017)
The results illustrated that the three-stage membrane process required the lowest treatment and cost and remained the most favourable option for all investigated prices while still achieving a helium recovery of 90.2 %. Scholes, Ghosh, and Ho (2017) state that membrane materials with even higher selectivities than polypyrrole, used in this investigation, do not further reduce treatment costs.
Extensive research has been undertaken to investigate the most favourable membrane materials for helium recovery from natural gas. The first helium extraction membranes included tubular silicate, quartz glass membranes, and polymeric membranes. Recently reported membranes include those constructed from ultra-microporous silica (Barboiu et al., 2006), molecular sieve carbons, porous graphene (Schrier, 2010), titanium silicates (Li et al., 2011), polyamides and mixed matrix membranes of polyimides and zeolitic imidazolate frameworks (Bernado, Drioli & Golemme, 2009).
Sunarso et al. (2016) surveyed five different membrane materials regarding their hydrogen and helium permeation performance and the related stabilities during permeation processes. Membranes evaluated included: silica, polymers, zeolite, metal-organic framework and mixed matrix membranes. The results illustrated that each membrane can be used for recovering helium, but it contains advantages and disadvantages in terms of permeability, selectivity, stability, cost, synthesis procedures, and reproducibility.
The literature on membrane materials performance shows that ultra-microporous inorganic membranes and glassy polymeric membranes are the most promising membranes for achieving successful helium recovery through membrane-based processes. Various polymeric membranes have been implemented successfully to upgrade crude helium to 90 % purity, which has energy duties comparable with other crude helium separation technologies (Scholes & Ghosh, 2017).
2 Challenges and outlook
2.1 Advantages and limitations
Table 5:Advantages and limitations of helium recovery technologies

2.2 Process of Helium Recovery from Natural Gas Review: Future Insights
The future success of membrane-based helium production from natural gas process technologies will depend on developing high-performance membranes and designing proper membrane separation processes for industrial applications. The focus must be on implementing patented low-cost modules for natural gas reserves to evaluate their operability and distinguish between the most optimal designs.
There are considerable opportunities to improve the efficiency of PSA processes for helium recovery through improved designs: optimised processing conditions by operating at, for example, cryogenic temperatures; and improvements in adsorbent materials (Rufford et al., 2014). A challenging approach is to develop helium-selective adsorbent materials, which can possibly reduce the size and energy requirements of PSA beds. Currently, no commercial adsorbents with sufficient helium capacity and selectivity are available to realise such an industrial application (Rufford et al., 2014).
There remains scope for developing and optimising various helium recovery process layouts featuring cryogenic distillation, PSA modules and membrane separation (Rufford et al., 2014). Although detailed technical feasibility on energy requirements and process economics of these integrated system designs still need to be conducted, it would appear that integration of the processes is key to providing the most economical solution for producing helium from natural gas. Such a process may include cryogenic distillation to recover helium from natural gas, coarse separation of crude helium using membrane technology, and final PSA purification.
3 Conclusions
Concerns regarding inequality between helium demand and production can be addressed by developing other improved helium recovery processes than the conventionally high capital-intensive cryogenic separation technologies.
To economically recover lower content helium (< 0.05 %) from natural gas and distinguish between optimal designs for Southern Africa’s helium-rich gas reserves, an integrated approach between cryogenic distillation for direct recovery of helium from natural and membrane-/adsorption-based processes for crude helium upgrading and purification must be designed.
Adsorption-based processes, excluding cryogenics, are not recommended for direct helium recovery from natural gas. Limitations were found for separating impurities and other gases from helium in low-quality gas streams, resulting in lower helium recoveries.
Although membrane-based technologies cannot yet compete with cryogenic separation for direct helium recovery from natural gas, there will be continual future interest and research developments due to the potential energy cost savings and lower associated process costs.
4 References
Agrawal, R, Herron, DM, Rowles, HC and Kinard, GE (2003), “Cryogenic Technology”, Kirk-Othmer Encyclopedia of Chemical Technology, Volume 8, John Wiley & Sons, Hoboken, NJ, 40-65.
Alders, M, Winterhalder, D and Wessling, M (2017), “Helium recovery using membrane processes”, Separation and Purification Technology, 189 (2017), 433-440.
Bakhsh, MSA, Jaynes, SE, Neu, BT, Smolarek, J and Emley, T (2007), Helium Recovery.
Baksh, MSA (2010), “Methods and systems for helium recovery”, US patent no. 2010/0251892 A1.
Barboiu, C, Mourgues, A, Sala, B, Julbe, A, Sanchez, J, de Perthuis, S and Hittner, D (2006), Desalination 200, 89.
Bernado, P, Drioli, E and Golemme, G (2009), Ind. Eng. Chem. Res. 48, 4638.
Crawford, D, Coyle, DA and Anantharaman, B (2010), “Method for nitrogen rejection and or helium recovery in a liquefaction plant”.
Daly, JW (2005), “Helium recovery from LNG.” International Petroleum Technology Conference, American Association of Petroleum Geologists (AAPG); the European Association of Geoscientists and Engineers (EAGE); the Society of Exploration Geophysicists (SEG); and the Society of Petroleum Engineers (SPE), Doha, Qatar, 1–4.
Häussinger, P, Glatthaar, R, Rhode, W, Kick, H, Benkmann, C, Weber, J, Wunschel, HJ, Stenke, V, Leicht, E and Stenger, H (2005), “Noble gases”, in: Ullmann’s Encyclopedia of Industrial Chemistry, G. Walter, editor, Wiley-VCH Weinheim, Baden-Wärttemberg, Germany.
Helium One (2017), “Technical Presentation”, June 2017.
Hosseini, SS (2009), “Membranes and Materials for Separation and Purification Hydrogen and Natural Gas, Department of Chemical Engineering and Biomolecular Engineering, National University of Singapore.
Kim, D. (2014), “Helium Extraction form LNG End Flash”, Department of Energy and Process Engineering, Norwegian University of Science and Technology.
Li, X, Zhou, C, Lin, Z, Rocha, J, Lito, PF, Santiago, AS and Silva, CM (2011), Microporous Mesoporous Mater. 137, 43.
Lindemann, U, Boeck, S, Blum, L and Kurtcuoglu, K (2010), AIP Conf. Proc., 1218, 271.
Mehrpooya, M and Shafaei, A. (2016), “Advanced exergy analysis of novel flash-based helium recovery from natural gas processes”, Energy, 114 (2016), 64-83.
Ritter, JA and Ebner, AD (2007), Sep. Sci. Technol. 42, 1123.
Rufford, TE, Chan, IK, Huang, SH and May, EF (2014), “A Review of Conventional and Emerging Process Technologies for the Recovery of Helium from Natural Gas”, Adsorption Science & Technology, 32 (2014), 49-72.
Scholes, CA and Ghosh, UK (2017), “Review of Membranes for Helium Separation and Purification”, Membranes, 7, 9.
Scholes, CA, Ghosh UK and Ho, MT (2017), Indust. Eng. Chem. Res. 56 (2017), 335-383.
Scholes, CA and Ghosh, UK (2016), “Helium separation through polymeric membranes: Selectivity targets”, J. Membr. Sci. 520 (2016), 221–230.
Schrier, J. (2010), J. Phys. Chem. Lett., 1, 2284.
Seader, JD and Henley, EJ (2006), Separation Process Principles, John Wiley & Sons, Hoboken, NJ.
Soleimany, A., Hosseini, SS and Gallucci, F (2017), “Recent progress in developments of membrane materials and modification techniques for high-performance helium separation and recovery: A review”, Chemical Engineering & Processing: Process Intensification, June 2017, URL http://dx.doi.org/10.1016/j.cep.2017.06.001.
Stern, SA, Sinclair, TF, Hareis, PJ, Vahldieck, NP and Mohr, PH (1965), Ind. Eng. Chem., 57, 49.
Sunarso, J, Hashim, SS, Lin, YS and Liu, SM (2017), “Membranes for helium recovery: An overview on the context, materials and future directions”, Separation and Purification Technology, 176 (2017), 335-383.
Tagliabue, M, Farrusseng, D, Valencia, S, Aguado, S, Ravon, U, Rizzo, C, Corma, A and Mirodatos, C (2009), Chem. Eng. J. 155, 553.
Victory, D, Miles, MW and Oelfke, RH (2009), “Helium recovery from natural gas integrated with NGL recovery”, Google Patents.
West, JE (2009), “Helium extraction and production techniques.” URL: (Accessed: January 27, 2011).

 To all knowledge
To all knowledge