1 Introduction
1.1 Exploration basics
Oil and gas are set to continue playing a vital role in meeting the world’s energy needs, accounting for nearly half of the primary energy mix in 2040, according to the Sustainable Development scenario put forward by the International Energy Agency [1]. There is no doubt that petroleum use and exploration are among the most potent driving forces shaping our modern world. This article conceptually describes the technical and economic aspects related to the use of isotopes for hydrocarbons exploration. Its purpose is to present the essential parameters that simply define this analytical tool. It should also help guide the reader on the decision mechanisms that an O&G Exploration and Production (E&P) company uses when drilling a well, carrying out a project to develop a reservoir, or investing in exploration.
Unlike a manufacturing industry, whose principal asset is the factory, which is not consumed while the company is producing goods, the primary value of an E&P company is its oil and gas reserves that it will consume as it carries on production. Therefore, the main objective of any E&P company will be to replenish the reserves consumed year after year so that they do not run out and the company does not shrink. On the contrary, it is desirable to increase resources when possible.
Consequently, the oil and gas sector needs to replenish the reserves put in production, and for this, it has several mechanisms: i) generation of reserves by exploration; ii) evaluation of probable and possible reserves in deposits already in production thanks to greater knowledge or a technological improvement; iii) generation of reserves by the application of secondary and assisted recovery techniques; and iv) acquisition of third-party reserves. From the means mentioned above, the first is the one that carries the most significant risk to the E&P company, since it involves assuming high investments in exploration with the chance of not achieving any commercial discovery. It should be noted that, by their high-risk nature, these investments are rarely fundable with external resources. The risk will be more significant in the case of the subsurface around the basin border zones, areas still unexplored, or areas where some exploratory activities have already been carried out without success.
1.2 Technical aspects of O&G exploration
Apart from finance, what aspects will an E&P company take into account in addressing an exploration project? First, it will look into the geology of the area and its location, assess the a priori risks presented by the basin and the rewards — discoveries — that could be obtained from it, and then define whether this upgrades its portfolio of exploration projects or not. If the area is considered attractive, the developer will acquire all the information available about it. By doing so, the firm prepares itself to compete and eventually obtain the rights to perform the block’s exploratory tasks.
Once the exploration permit has been issued, geologists will complete the current information with surface, gravimetric, magnetometric, and geochemical survey studies, among others. In general, any crude oil correlation or characterisation study depends upon using a multitude of parameters. These may be bulk parameters such as API gravity, elemental ratios such as Ni/V ratios, stable isotopes, or more specific biomarker parameters. In general, the more parameters used for this purpose, the more confident one can be of the outcome.
If considered worth it, they will also commission a seismic study that should provide a better understanding of the bedrock. After these steps, and if the results are conducive, the company must make the most critical decision in this exploratory venture, whether or not to drill a well [2]. To proceed onto the drilling phase, the developer must verify that the prerequisites for hydrocarbon accumulation and formation of oil or gas deposits are accomplished, i.e. that the following phenomena have taken place: trap, seal, timing, and migration.
Suppose the well was found to be an oil or gas discovery. In that case, other wells, called appraisal wells, are likely to be drilled to understand the extent and size of the accumulation, quantify the magnitude of the discovery and plan its initial development. Therefore, hydrocarbon exploration has become more and more sophisticated over the years, with some new techniques being developed and applied to improve success rates.
So, our primary motivation is, can we come up with ways to develop exploration tools which are maybe better equipped to finding petroleum deposits? And are we able to provide better detail about known occurrences?
1.3 Why are isotopes useful?

Figure 1. Hydrogen two naturally occurring isotopes, deuterium and tritium. Source: https://www.energy.gov/science/doe-explainsisotopes
The nucleus of an atom encases protons and neutrons. While the number of protons designates the element, the number of neutrons defines that element’s isotope (Figure 1). For instance, most carbon (≈ 99 %) has six protons and six neutrons, however, about 1 % of the carbon in the Earth’s biosphere has six protons and seven neutrons (13C) materialising one of its isotopes. There are stable and radiogenic isotopes. 13C and other stable isotopes do not decay into other elements. In contrast, radioactive isotopes such as 14C are unstable and will decay into other elements [3].
The stable isotope concentrations of a compound are displayed as the molar ratio of the heavy-to-light isotopes. Since this quotient is small, specialists typically present stable isotope abundances relative to an international standard using delta (δ) notation as:
δX = (Resample/Standard – 1) x 1000, ‰
where δX is the relative abundance or delta value of the sample for element X in parts per thousand (‰) and R is the molar ratio of the less common isotope to the more common isotope, both for the sample and an international standard [4].
Isotopes are employed as tracers of origin and processes in the most varied fields, such as ecology, food science, archaeology, and forensic sciences. In the Earth Sciences, isotopes have found immense niches in geochronology and proxies for the sources and ancient chemical conditions of the environment where rocks were formed, i.e. hydrosphere, biosphere, tectonic and climate evolution. Moreover, the analysis of specific samples in comprehensive stratigraphic packages permits the assessment of vertical variations of the isotopic proxies, which can reflect ancient geochemical conditions that are in turn complexly interlinked to hydrocarbon accumulation. Compound-specific isotope analysis (CSIA) is a powerful tool in petroleum exploration. Combining other geochemical approaches can provide evidence of the source, charge history, maturity, and reservoir processes, thus supporting field development planning and exploration.
Two common exploration questions are the timing of emplacement and the origin of global hydrocarbon accumulations. Interpreting organic matter types and depositional environments provide a far better understanding of the quality and quantity of oil and gas produced from a particular kind of source material. To achieve this, E&P use sophisticated techniques that take account of isotope ratios to correlate oils, or gases, with their suspected sources.
1.4 Analytical determination of isotopes
The utilisation of isotopes in petroleum exploration has more recently evolved due to the development of the Gas Chromatograph-Isotopic Ratios Mass Spectrometer (GCIRMS) system.
The sample is injected into the GC, where compounds are separated on a capillary column and then converted to CO2 in the reactor, using a source of O2 and a catalyst. The CO2 gas is then transferred into the mass spectrometer, where it is ionised. In carbon isotopes, the δ13C values of individual compounds are calculated relative to either a reference gas or a co-injected compound with a known isotopic δ13C value. δ2H measurements require a pyrolysis reactor to generate H2 gas; organic compounds are carried from the GC column through a high-temperature conversion reactor; reduced H gas is then transferred to the mass spectrometer.
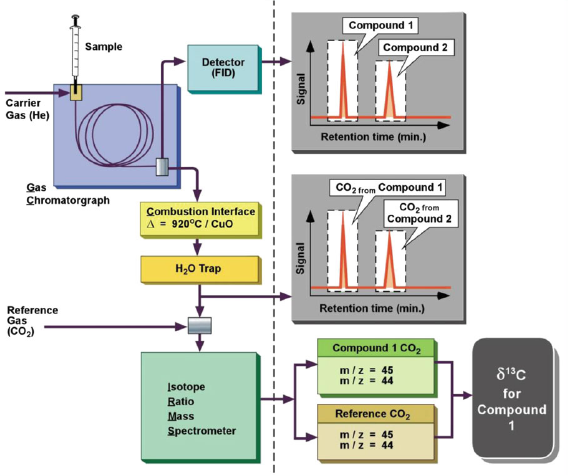
Figure 2. Schematic representation of a GCIRMS system for continuous flow determination of isotopic compositions of individual compounds. Source: https://doi.org/10.1007/978-3-642-10637-8_31
Gas may be considered today as a reliable tracer for the understanding of associated liquid hydrocarbons. Isotopic determinations of stables isotopes and noble gases offer important hints to reconstruct the geological history of hydrocarbons. Recent analytical advances in carbon isotopes of natural gases (methane, butane, carbon dioxide) due mainly to the development of GC-IRMS have allowed tracing back some of the physicochemical processes affecting natural gas chemical signatures. GCIRMS has also become particularly useful for the characterisation of groundwater contaminants.
2 Isotope Case Studies
Petroleum geoscientists relatively low-cost, high-throughput bulk data to screen for source rock quality and thermal maturity. More in-depth analytical techniques are used in the context of full fluid and reservoir properties. Geologists may be able to correlate source rocks and reservoir oils, to understand in-reservoir processes, such as the biodegradation of in-reservoir oils, and determine fluid generation and migration history, including existing reservoir connectivity. These resources are preeminently powerful when coupled with other measurements made during the exploration and development process. Compositional analysis during drilling, downhole fluid analysis and other wireline measurements, pressure, volume, temperature and chemical analyses are examples.
The two most commonly used stable isotopes in hydrocarbon exploration are carbon and hydrogen; there have been occasions where stable sulfur isotopes have been used. The natural abundance of the stable isotopes commonly utilised in exploration and the international standards used in the calculation of their δ values are given in the following table:

For the successful application of stable isotopes in exploration, the core aspect is based on variations in the isotopic compositions of the compounds being monitored. But why do we see these variations? The answer to that question can be deducted from photosynthesis and the formation of the source material for crude oils.
Early examples of the application of stable carbon isotopes are found in the fields of:
- gas exploration, where E&P companies redirected exploration efforts after source rocks of gas deposits were identified by 13C/12C analyses of methane
- wildcat drilling, in which the carbon isotope composition of methane from the headspace of canned cuttings typifies autochthonous methane and offers evidence on the maturity of organic matter with depth;
- oil/oil and source-rock/oil correlation, where the ‘isotopic type curve technique’ — a correlation method — was extensively applied to exploration problems in the British North Sea region. More recent case studies are described in the following sections.
2.1 Carbon
C3 and C4 plants for differentiating source materials
Carbon exists as three isotopes, 12C, 13C, and 14C. While 12C and 13C are stable isotopes, 14C is a radioactive isotope with a half-life of 5,730 years that has been widely used in recent years in many biofuel studies. The degree of fractionation between atmospheric 13CO2 and 12CO2 will vary during photosynthesis depending on whether the source material is land plant or marine species. Carbon isotopes are often used to distinguish between oils derived from land and aquatic organic matter. What is more, various photosynthetic cycles within the first group will further affect the extent of fractionation.
C3 plants generally grow in cooler wetter climates. They include shrubs and trees with isotope values in the 26 to 30 ‰ range, plants with the C4 cycle, including grasses, corn, maise, grow under dryer and hotter conditions and have isotope values in the 10 to 14‰ range [5]. Isotopic variations in the original plant source materials are one reason for variations in the resulting crude oils.
C isotopic fractionation
Processes such as maturation and biodegradation can affect the isotopic composition of crude oil beyond variations in isotopic source signatures. There are two fractionation processes responsible for these changes, namely the kinetic effect and the equilibrium effect. While both phenomena may be active, in most hydrocarbon exploration studies, it is primarily the essential kinetic isotope effect, most evident in natural gas exploration studies.
Decades ago [6], it was observed that petroleums could be genetically classified through covariance patterns of their compound classes and that C and H isotopes in methane were genetically controlled. Carbonate and shale sourced petroleums may be additionally differentiated through their deuterium (2H) content. Bacterial methane in freshwater environments was found to be characteristically depleted in deuterium; even complex geneses of natural gases could be unravelled using compositional and isotopic signatures. New analytical developments led to the application of carbon isotope analyses of gaseous hydrocarbons desorbed from sediments for geochemical surface exploration.
The recent discovery of two thermogenic oil seeps 70 km apart onshore Jamaica constitutes an example of using oil fingerprinting, gas chromatography-mass spectrometry and stable isotope ratio distributions. The finding suggests that a previously unrecognised hydrocarbon system may well be present. Also, exploration activities taking in place in the Caribbean plate remarked that the three broad elements comprising the essence of the structure — the Lower Nicaraguan Rise, or Siuna Terrane; the Upper Nicaraguan Rise, or Chortis; and part of the Great Caribbean Arc or northern periphery of the Caribbean plate or platelets — can also be identified through petroleum geochemistry. For instance, Windsor #1 well in the north of Jamaica was drilled in terrain with strong affinities with the Yucatan geology in Mexico [7].
Hydrocarbon generation and expulsion from deep asphalt cracking
Analysis of the composition and carbon isotopy of a reservoir and examining the regeneration hydrocarbon problem are essential to exploring or developing oil and gas.
If damaged, paleo-reservoirs may produce asphalt, and asphalt may be cracked under high temperature to generate lighter hydrocarbons. Researchers from the Guangzhou Institute of Geochemistry and the China National Petroleum Corporation [8] gathered asphaltic sand formed by biodegradation in the Silurian from the Tarim Basin in Northwest China and investigated the effects of heat on the compositions, isotopes, and physical properties of the sand using a high-pressure reaction vessel (Figure 3). The asphaltic sand produces gas at high temperature. The cracked gas has lighter carbon isotopes: there are fewer compositions of C6+ and above, and it is dominated by light oil; the gas yield increases substantially after 400 °C and reaches its peak at 550 °C.
The main takeaways from this application are: First, secondary thermal stress can change the composition, carbon isotope and structure of bituminous sandstones. And second, hydrocarbon regeneration will not form large-scale crude oil but light oil and gas.

Figure 3. Curves of n-alkane carbon isotopes under different experimental temperatures. Source: https://link.springer.com/article/10.1007/s12182-015-0014-0/figures/40
2.2 Hydrogen
Similarly, the hydrogen CSIA of n-alkanes provides information about the extent of thermal maturation and other physical processes. Oils show increasing δ2H values with increasing C number, reflecting thermal maturation. Condensates also offer the same trend, but with even higher δ2H values. A thorough recently published study on the Barents Sea provides an excellent case study on how to use CSIA to improve the understanding of the source origin of oil families, and how this approach can support long-term field exploration, development planning, and potentially production monitoring.

Figure 4. The main factors controlling C and H isotope compositions of n-alkyl lipids in the source rock and petroleum reservoir. Source: https://core.ac.uk/download/pdf/146460589.pdf
Organic matter formation
Two key factors play a role in this phenomenon, concerning both aquatic and terrestrial-derived organic compounds, including n-alkanes: i) the 2H/1H composition of source water for the organism, and ii) the physiological and biochemical processes involved in fixing water-derived H into organic compounds. The δ2H values of source water for plants are initially determined by the 2H/1H composition of meteoric precipitation. Compound-specific H isotope studies have revealed that biosynthetic 2H/1H fractionation can lead to 2H-depletion relative to source water by 100–250‰.
One aspect to highlight is the importance of understanding the complex interplay among the environmental and organism-specific physiological and biochemical processes that control δ2H values of organic compounds synthesised by extant biota.
Organic matter maturation and petroleum generation
The primary environmental/biological C and H isotope signature of n-alkyl lipids is further modified by thermal maturation and hydrocarbon expulsion during petroleum generation. From a petroleum geochemist’s viewpoint, key processes and reactions start when kerogen cracking, bitumen formation and petroleum fluid expulsion occur. During these processes, and as a result of kinetic isotope effects, a C-bound H can undergo further 2H/1H fractionation.
The primary common outcome of some research in this field is that kinetic models can be used for qualitative prediction of 2H/1H fractionation during kerogen/oil cracking in natural settings. The kinetic isotope effects are likely to be significant at thermal maturities of vitrinite reflectance (Ro) > 1.5.
Biodegradation
Subsurface biodegradation leads to a well-characterised sequence of compound class losses as anaerobic microbial groups metabolise hydrocarbons. Biodegradation preferentially removes 12C and 1H, but H isotope compositions appear less conservative due to biodegradation. Variations of up to 35‰ have been observed [9], and stable H isotopes are potentially useful in understanding and even quantifying the biodegradation effect.
2.3 Sulfur
Sulfur has tended to be considered a contaminant rather than an integral component of petroleum, yet, sulfur isotopes’ capability to elucidate many evolution problems, migration, and oil alteration have been demonstrated.
With Exxon Mobil Exploration Company’s support, researchers from Israel and the US proposed comparing the δ34S values of benzothiophenes and dibenzothiophenes to detect thermochemical sulfate reduction alterations of oils from the very early stages up to highly altered oils [10]. This approach should find numerous uses in petroleum exploration and understand the basic reaction mechanisms and kinetics of thermochemical sulfate reduction and secondary sulfur incorporation into oils.
GC-MC-ICPMS analyses revealed a wide distribution of δ34S values for individual organic sulfur compounds in oils from various Gulf of Mexico oil fields. The maximum difference between individual compounds within the same oil reaches up to 25‰. The oils can be divided into two groups: those with more negative δ34S values and a little variation between individual compounds. Those with more positive δ34S values and large differences between individual compounds.
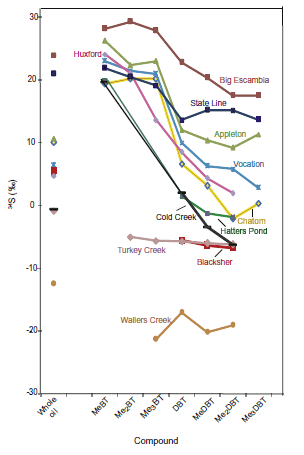
Figure 5. δ34S values of individual organosulfur compounds from whole oils sourced in the Smackover Formation, Gulf of Mexico. Source: http://dx.doi.org/10.1016/j.gca.2012.01.023
2.4 Rhenium-osmium
The rhenium-osmium (Re-Os) geochronometer is an increasingly documented tool for estimating depositional ages of organic-rich rocks, which are likely associated with hydrocarbon potential. The decay of 187Re to 187Os is a useful chronometer and tracer, and both elements are highly enriched in black shales, which are the crustal source rocks of many crude oils. Sufficient amounts of these elements are present in hydrocarbons to make the radioisotope system viable to approximate the timing of emplacement, possibly the source, of migrated oils and their biodegraded products oil sands. Thus, some researchers believe this approach should apply to dating oil deposits worldwide.
Assigning accurate geochronological constraints on organic-rich horizons is central to understand the nature of the depositional environment and fossil hydrocarbon system in systems such as the Sao Francisco Basin in Brazil [11], the Amundsen Basin [12] and the giant oil sand deposits of Alberta [13], both in Canada, and the Staffin Shale Formation in Isle of Skye, UK [14]. Re-Os records from migrated hydrocarbons, for example, establish the timing of petroleum emplacement for the oil sand deposits of Alberta at around 112 million years ago. Likewise, most data from various deposits within the system show similar characteristics, supporting a single source’s hypothesis for this giant structure.
2.5 Rubidium-strontium
Strontium isotope stratigraphy (SIS) offers petroleum exploration companies and researchers the opportunity to develop a more detailed sedimentation history of petroleum‐bearing basins, enabling them to determine drilling targets and potential reservoirs in exploration accurately.
Additionally, pyrite rubidium-strontium (Rb-Sr) isotope mass spectrometry is a well-developed technique for dating petroleum systems by hydrocarbon or associated authigenic minerals. The precipitation of authigenic pyrite in petroliferous basins is commonly genetically associated with hydrocarbon generation, migration, accumulation, or destruction, all of which are essential data for petroleum system analysis and hydrocarbon exploration.
2.6 Neodymium
Neodymium could be suitable for oil-source rock correlations. Several studies showed that its isotopic equilibrium might occur between authigenic clay minerals, formation waters, source rocks, and petroleum. For instance, 143Nd/144Nd ratios from bitumen, crude oil, and kerogen in China contributed to finding that petroleum generation was associated with fluid action from deep sources, including the mantle, lower crust, and buried strata in rift basins and buried foreland basins at craton margins or subduction zones [15].
3 Other Isotope Applications Related to O&G
3.1 Gas exploration
Natural gas exploration has become as critical as the search for liquid hydrocarbons in the last decade. Although we could take advantage of a large part of the R&D work performed for petroleum exploration to understand gas deposits, it rapidly looked like some specific issues were to be addressed separately.
Several new applications of this association have been presented since the 2000s: a new way for distinguishing bacterial and thermogenic gas origins, the characterisation of the parameters related to the genesis of thermogenic gas: primary versus secondary cracking, the openness of the system, relations between gas isotope signatures and biodegradation.
Carbon isotopes
Bacterial contamination is an important issue when characterising the gas origin and migration with stable isotopes. It has been long known that bacterial genesis gave gas enriched in methane, isotopically light compared to a thermal generation. This distinction is based on both the dryness of bacterial gas and on the carbon isotopic ratio of methane being lighter for this type.
Another diagram using both the chemical and isotopic signatures of ethane and propane was developed to better characterise the gas’s maturity. For an acceptable interpretation of data in Figure 6, one essential parameter to consider is the system’s degree of openness. The retention (or not) of the hydrocarbons in the generation area drastically affects the maturity trends. This is primordial when characterising the composition of accumulated fluids; indeed, as shown below, the same source rock and the same reservoir that gathers all the hydrocarbons generated will be filled with oil and gas. In contrast, a reservoir that accumulates only the last part of the hydrocarbons produced will only be filled with dry gas. In other words, a late accumulation may lead to a gas highly enriched in nitrogen in overmature source rocks.

Figure 6. General scheme of hydrocarbon and N2 generation from organic matter.
Noble gases
Among other new methodologies, noble gas coupled to stable isotopes is a cutting-edge tool. Their chemical inertia enables their use as specific tracers of sources and associated physical processes [16]. In addition to petroleum exploration applications, and because of their chemical inertia, noble gases can be used to track the output of multi-layered or compartmentalised fields, the relative proportions derived from each field block vs time. This kind of new geochemical monitoring of a multi-play gas field may give precise and less expensive information about the production evolution.
Helium, Neon, Argon, Krypton, Xenon (He, Ne, Ar, Kr, Xe) contain isotopes generated through natural nuclear reactions occurring in the minerals. Some isotopes are radiogenic while others are called nucleogenic as their generation occurs through atomic reactions as (α,n) or (n,α) on nuclei of Lithium, Magnesium, Fluorine, Oxygen (Li, Mg, F, O). The absolute amount of the so-generated isotopes increases linearly with time for time ranges compatible with petroleum systems, i.e., several hundred million years.
Given that some isotopes (e.g., 4He, 40Ar) are produced by natural radioactivity, they may well represent geochronometers, providing a quantitative estimation of hydrocarbons’ residence times a reservoir. For instance, their properties led to a tool for a tentative quantification of the proportion of hydrocarbon gases leaked from a reservoir. The distinction of natural gas origin (bacterial vs thermogenic) may also be confirmed by a novel methodology using these two radiogenic isotopes’ apparent production ratio.
3.2 Environmental Forensics
Over the past decade, methods developed and used for a long time in hydrocarbon exploration and exploitation have made their way to the forefront of environmental forensics. Whereas exploratory ventures are geared towards finding new resources, environmental forensics is directed to looking at what happens to those resources, or products derived from them, are spilt into the environment. The source may be a tanker, pipeline, underground storage tank or similar infrastructure.
Two major environmental forensics practices have developed: precise source/contaminant correlation and monitoring the onset of natural attenuation. Both of these applications can be utilised in groundwater studies. Another successful application of the aforementioned noble gas isotopes consists of using them as inert tracers of leakage from the reservoirs through aquifers. The fundamental behind these applications is that variations in the contaminant’s isotopic composition may provide additional evidence that can be used to differentiate the sources. The utilisation of 2 or 3 isotope values (e.g., C, H, Cl) provides an even more powerful discriminatory tool or fingerprint. As a plus, various isotope hydrology techniques can monitor the impact of fracking on ground and surface water.
Isotope hydrology can distinguish substances that can occur naturally in groundwater, but whose presence can also result from pollution. New groundwater age radiotracers like 81Kr and argon isotopes will help decide how long contaminants linked with fracking and oil and gas production may reside in drinking water aquifers [17]. We are also seeing increasing interest and use of the stable isotopes of chlorine and nitrogen.
In 1984, a consultant showed that the water compositions from various formations were very distinct across southern Ontario [18]. His work then inspired the Laboratory for Stable Isotope Science at The University of Western Ontario to build on that distinction he was describing for oxygen and hydrogen isotopes. Now, there’s an extensive database of shallow and deep formation water and gas isotopic compositions readily available for this Canadian region. It provides a water fingerprint for bedrock formations and the gas fingerprint for hydrocarbon reservoirs. It can be used to trace the origin of leakage from orphaned wells and for all other kinds of purposes [19].
3.3 Anthropogenic lead in petroleum
Tetraethyl lead is still used in some grades of gasoline but is being phased out on environmental grounds. The ALAS model is a tool for estimating the age of hydrocarbon release [20]. It is based on systematic increases in lead (Pb) isotope ratios in petrol caused by changes in Pb ores sources used by the petroleum industry in North America. The model calibration curve relies on isotope data of soils and water of known age, affected by petroleum products, and measurements of the hydrocarbon products themselves.
Mississippi-Valley-Type Deposits (MVT) are zinc and lead deposits in carbonate sedimentary rocks that account for approximately 25 per cent of the world’s lead and zinc resources. They are so-named because several classic MVT districts are located within the Mississippi River’s drainage basin in the central United States and other significant Canadian communities. Due to increasing radiogenic MVT type of ores in leaded petrol, age uncertainties are as low as a few years. This model is used to compare environmental releases of diesel and motor oil, for example, to their sources, and anthropogenic gasoline-derived lead has been mapped for many areas in North America.

Figure 7. Tectonic controls of Mississippi Valley-type lead–zinc mineralisation. Source: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/mississippi-valley-type-deposit
4 Conclusion
The genetic identification of different types of petroleum is vital for assessing its sources and exploration potential. Its chemical and isotopic compositions vary significantly due to the complexity of its generation, migration, and accumulation processes.
Recent analytical developments using naturally occurring isotope tracers in hydrocarbons, high-resolution data sets of natural gases and associated fluids from the surface to target reservoirs, and the incorporation of noble gas geochemistry and microbiology into more traditional geological and geochemical approaches offer powerful analytical tools for identifying the sources of petroleum accumulation.
An emerging and complex isotopic method, allows oil and gas companies and scientists to probe the molecular position of hydrogen isotopes in the methane gas relative to its single carbon atom, giving new diagnostic insights into which gas reservoirs the suspected stray gases may have come from. This method may also facilitate the distinction of deep thermogenic methane from naturally-produced methane by soil bacteria. Some of the isotope approaches developed to perform oil and gas exploration have broader applications, including detecting contamination in groundwater, air, and soil.
Cutting-edge techniques have always been utilised in petroleum exploration to reduce costs and improve efficiencies. If society moves towards lower-carbon energy systems, advances in isotope analytical methods will continue to play a vital role in the industry going forward.
5 References
[1] https://www.iea.org/reports/world-energy-outlook-2020
[2] Stinco, L. (2013). Aspectos técnicos, estratégicos y económicos de la exploración y producción de hidrocarburos. 1a ed. Buenos Aires. Instituto Argentino del Petróleo y del Gas.
[3] https://www.energy.gov/science/doe-explainsisotopes
[4] http://www.uwyo.edu/sif/stable-isotopes/isotope-ratio.html
[5] Philp R.P., Monaco G.L. (2012) Applications of Stable Isotopes in Hydrocarbon Exploration and Environmental Forensics. In: Baskaran M. (eds) Handbook of Environmental Isotope Geochemistry. Advances in Isotope Geochemistry. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10637-8_31
[6] Schoell, M. (1984). Recent advances in petroleum isotope geochemistry, Organic Geochemistry, Volume 6, Pages 645-663, ISSN 0146-6380, https://doi.org/10.1016/0146-6380(84)90086-X
[7] http://cmi-capital.com/blog/first-thermogenic-oil-seeps-discovered-onshore-jamaica/
[8] Gong S, Peng PA, Lu YH, et al. (2004). The model experiments for secondary pyrolysis of biodegraded bituminous sandstone. Chin Sci Bull. 49:39–47.
[9] Sun, Y., Chen, Z., Xu, S.& Cai, P. 2005. Stable carbon and hydrogen isotopic fractionation of individual n-alkanes accompanying biodegradation: evidence from a group of progressively biodegraded oils. Organic Geochemistry, 36, 225–238.
[10] Amrani, A., Deev, A., Sessions, A. L., Tang, Y., Adkins, J. F., Hill, R. J., … & Wei, Z. (2012). The sulfur-isotopic compositions of benzothiophenes and dibenzothiophenes as a proxy for thermochemical sulfate reduction. Geochimica et Cosmochimica Acta, 84, 152-164.
[11] Bertoni, M.E., et al. (2014). Neoproterozoic Re-Os systematics of organic-rich rocks in the Sao Francisco Basin, Brazil and implications for hydrocarbon exploration. Precambrian Res. 255, 355–366. Elsevier
[12] van Acken, D., Thomson, D., Rainbird, R.H., Creaser, R.A.(2013). Constraining the depositional history of the Neoproterozoic Shaler Supergroup, Amundsen Basin, NW Canada: Rhenium-osmium dating of black shales from the Wynniatt and BootInlet Formations. Precambrian Res. 236, 124–131.
[13] Selby, D., Creaser, R.A. (2005). Direct radiometric dating of hydrocarbon deposits using rhenium-osmium isotopes. Science 308, 5726, 1293–1295.
[14] Selby, D. (2007). Direct Rhenium–Osmium age of the Oxfordian-Kimmeridgianboundary, Staffin Bay, Isle of Skye, UK, and the Late Jurassic time scale. NorskGeologisk Tidsskrift 87 (3), 291–299.
[15] Bing-Quan, Z., Jing-Lian, Z., Xiang-Lin, T., Xiang-Yang, C., Cai-Yuan, F., Ying, L., & Ju-Ying, L. (2001). Pb, Sr, and Nd isotopic features in organic matter from China and their implications for petroleum generation and migration. Geochimica et Cosmochimica Acta, 65(15), 2555-2570.
[16] Prinzhofer, A., & Battani, A. (2003). Gas isotopes tracing: an important tool for hydrocarbons exploration. Oil & gas science and technology, 58(2), 299-311.
[18] https://www.geosyntec.com/people/peter-dollar
[19] Skuce, M., Longstaffe, F.J., Carter, T.R., and Potter, J. (2015) Isotopic fingerprinting of groundwaters in southwestern Ontario: Applications to abandoned well remediation. Applied Geochemistry 58: 1-13.
[20] Hurst, R.W. (2000). Applications of anthropogenic lead archaeostratigraphy (ALAS model) to hydrocarbon remediation. Environ. For. 1, 11-23.

 To all knowledge
To all knowledge