1 Introduction
1.1 Biomass
Biomass refers to non-fossil material of biological origin, such as energy crops, agricultural and forestry wastes and by-products, manure, or microbial biomass [1]. Biomass energy, or energy derived from living things, has been used by people ever since the early “cave man” lit wood fires for warmth or cooking. Today, biomass is used to power machines like electric generators.
The energy in biomass is initially obtained from the sun: plants transform carbon dioxide and water into nutrients (carbohydrates) through photosynthesis. Both direct and indirect methods can be used to convert the energy from these creatures into usable energy. Direct combustion of biomass can provide heat, direct conversion of biomass to electricity, or direct conversion of biomass to biofuel (indirect).
Until the middle of the 1800s, biomass accounted for most of the total yearly U.S. energy consumption. Biomass is a popular fuel in many nations, particularly for heating and cooking in underdeveloped countries. In many industrialised nations, the use of biomass fuels for electricity production and transportation is rising to reduce carbon dioxide emissions from burning fossil fuels. Nearly five quadrillion British thermal units (Btu) and 5% of all primary energy used in the United States were provided by biomass in 2021.

Figure 1. Primary biomass feedstocks comprise (top row) switchgrass, copra (coconut), cotton, and jatropha; (middle row) municipal solid waste (MSW), sunflowers, palm nuts, and canola; (bottom row) wheat, sugar cane, wood, and rice. Source: https://education.nationalgeographic.org/resource/biomass-energy
Lignocellulosic biomass is the most plentiful renewable carbon source on Earth. Obtainable sources of biomass include forest residues, crop residues, purpose-grown energy crops (e.g., grasses), food wastes, and animal manure. These materials are the stringy structural parts of plants and are made mainly of cellulose, hemicellulose, and lignin. Compared with so-called first-generation organic feedstocks such as vegetable oils, sugars, and starches, nature has made these parts of the plants challenging to disassemble into chemical building blocks, making usage of this carbon source a challenge for engineers and scientists.
Many alternate fuels are available to replace the big three—coal, oil, and gas. These include biomass, particularly wood, bagasse, fast-growing switch grass, agricultural feedstocks, and used cooking oil. Target products include advanced hydrocarbon biofuels that are indistinguishable from fossil-based gasoline, diesel or jet fuels, along with bio-based chemicals and materials. To make renewable bio-products derived from biomass economically competitive with those made from fossil fuels, technologies must be developed to convert this renewable supply of carbon more effectively.
1.2 Anaerobic Digestion
Anaerobic digestion (AD) is a natural biological breakdown process that occurs in oxygen-free conditions. It means the conversion of organic matter by microorganisms to yield a gaseous product, known as “biogas,” leaving a more balanced solid product, known as “digestate.” AD is one of the most practical biological conversion processes of biomass into biofuel [2]. It breaks down organic matter by microorganisms and enzymes in an oxygen-free environment to produce biogas.
AD occurs in some natural environments, such as marshlands, sediments, and the digestive tracts of termites and ruminants; methane is generated from all these. Yet, suitable conditions can also be created artificially in digestion tanks and landfills. AD has been used for over a century in sewage treatment and, more recently, to treat designated farm and industrial wastes.
Due to the depth and compaction of the waste, modern landfills also develop anaerobic conditions, thus generating a fluid known as “landfill gas.” The feedstock is usually unsorted municipal solid waste, often mixed with construction, industrial and other unwanted refuses. The stabilisation process is prolonged, and little control is achievable. Proof of acceleration in long-term, large-scale landfill trials is eagerly sought. In contrast to digesters, landfills have quite different conditions, so the discussion is typically restricted to the former.
AD first called attention in the 1970s as a renewable energy source, directly after the “energy crises” of the juncture, and also as a means of stabilising municipal solid waste. Modern drivers include the increasing levels of waste generation and the problems associated with landfilling, such as groundwater pollution and methane migration. The development of AD was a natural result of the many years of expertise gained from the digestion of agricultural and sewage sludge, and it is today a recognised, dependable method for treating municipal solid waste.
1.3 Gasification
Biomass gasification is one process by which biomass can be converted into value-added products, therefore mitigating polluting waste disposal strategies whilst simultaneously generating valuable products such as biochar, syngas, power, heat, bio-fuels, and fertiliser. It entails the conversion of biomass into a mixture of combustible gases, primarily carbon monoxide (CO), nitrogen (N2), methane (CH4), and hydrogen (H2). Gasification occurs under controlled oxidation of biomass at high temperatures ranging from 800 to 1100 °C.
Gasification produces syngas, an intermediate in generating synthetic natural gas, ammonia, methanol, and other value-added chemicals. Syngas contains approximately 40–70% H2, 15–25% CO, and 1–2% CO2, having a calorific value of 4–5 MJ/m3. It can be used straightforwardly as a fuel in internal combustion engines, for thermal applications, or to produce mechanical or electrical power.
The last 20 to 30 years have seen increased interest in biomass gasification. Gasification technology has undergone extensive development to be used, made market-ready, and developed into a mature, practical, but also competitive technology. The variety of the gases produced is the primary factor driving interest in biomass gasification. Gasification still faces difficulties like tar or other trace impurities, which present operational issues for downstream gas usage compared to the tried-and-true combustion technique. Examples of this type of gas utilisation include the employment of catalysts to reform flammable gas as well as gas engines and turbines.
1.4 Pyrolysis
Pyrolysis can be explained as the decomposition of a molecule by heat within a nonreactive atmosphere. It is a process by which a solid (or occasionally a liquid) undergoes thermal conversion into smaller volatile molecules without reacting with oxygen or any other oxidants. Pyrolysis is an essential process for the thermogenesis of most solid fuels. Pyrolysis of a given material can produce many different combustion derivatives, called pyrolysis products.
Pyrolysis converts biomass to solid (biochar), liquid (bio-oil), and non-condensable gases (H2, CH4, CO, CO2, N etc.). Compared to gasification, it takes place at higher temperatures —in the range of 300–800 °C— in the absence of oxygen. The composition of pyrolytic products depends on the process conditions: temperature and residence time. Using slow kinetics, biochar production can be maximised, which is preferred for agronomic applications. By employing the flash pyrolysis process, more bio-oil can be produced to run combustion engines.
There are some problems relating to bio-oils corrosion and stability as it starts undergoing degradation after the pyrolysis is over. Oil upgradation may be required in some instances; it can be done by reducing the oxygen content and eliminating the alkalis through catalytic cracking and hydrogenation.
It is essential to understand that pyrolysis is not a phase change; it is a chemical process. More correctly, it is a thermal degradation process, as it occurs under heat and degrades larger molecules into smaller ones [3].
2 Applications
2.1 Biomass As A Fuel
As conventional energy prices rise, biomass fuels are becoming a more common alternative to fossil fuels. The primary biomass fuel is wood waste; however, bagasse (sugar cane residue), ag-fuel, or fuels derived from straw, rice hulls, and shell hulls, biomass grown as a fuel crop (e.g., switch grass), and other agricultural sources are becoming more popular. Plywood, lumber, OSB (oriented strand board), and related plants have long used bark, wood waste, planer shavings, and sander dust from plant operations to provide process heat for drying and pressing boards rather than fossil fuels. However, biomass fuels are finding applications in nonforest products industries as well.
Biomass fuels replace up to 5-10% of coal during cofiring in some utility boilers at power plants. The reason for replacing only a portion of the fossil fuel is that generally, wood fuels have a lower heating value and higher moisture content so that only a part of coal fuel can be replaced without significant loss of boiler performance and output. The advantages of biomass are lower fuel cost, and emissions since wood fuels are much lower in sulphur content than most typical coals. They also contain ash, which has alkali components that can react with and remove some sulphur dioxide.

Figure 2. Panoramic view of the Hazlehurst Wood Pellets Plant in Georgia, US. Source: http://www.framfuels.com/mills.cms
In addition, in those countries that have endorsed the Paris Agreement, the use of naturally derived fuels, as opposed to fossil fuels, is a standard method to reduce the emission of “greenhouse gases” and obtain CO2 emission credits since renewable energies from plant sources are considered “carbon neutral” under this accord. This is common in Europe and is an emerging market in itself, with wood pellet producers in the United States shipping their products across the Atlantic. Fossil fuels are burdened with associated CO2 greenhouse gas emissions.
Although company-wide studies can cost thousands of dollars to count direct and indirect CO2 emissions when it comes to burning a fuel, the math is quite simple: Multiply the fuel weight per year by the carbon fraction, then multiply by 3.66 (3.66 is the molecular weight of CO2 divided by that of carbon) and divide by 2000. This result is the tpy (tons/yr) of CO2 generated. The carbon fraction in the fuel can be found in reference texts or via lab work. Basic chemistry can also be used to calculate the carbon fraction. For example, the carbon weight for methane is 12; when divided by the total weight of 16, the result is 0.75, or 75% carbon.
2.2 Biogas & Biomethane
Microorganisms degrade biodegradable material during a set of biological processes known as AD that occurs without oxygen. One of the final outputs is biogas, which may either be converted into renewable natural gas and transportation fuels or burned to produce electricity and heat.
Food waste, cattle manure, municipal wastewater solids, fats, oils, and grease, industrial wastewater and residuals, and numerous other organic waste streams are continuously being converted into biogas using a variety of anaerobic digestion processes. Separated digested solids can be used to make various products, compost, dairy bedding, or direct application to crops. The digestate, which contains nutrients, can be utilised as fertiliser in agriculture.

Figure 3. The multiple production pathways for biogas and biomethane. Source: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/an-introduction-to-biogas-and-biomethane
To make insoluble organic polymers like carbohydrates available to other bacteria, the materials are first subjected to bacterial hydrolysis. Acidogenic bacteria subsequently convert sugars and amino acids into carbon dioxide, hydrogen, ammonia, and organic acids. The latter are then transformed into acetic acid by acetogenic bacteria along with extra ammonia, hydrogen, and carbon dioxide. The compounds are finally converted into methane and carbon dioxide by methanogens.
Commercially available anaerobic digester systems come in a wide variety. The following are a few of them:
- Complete mix digester — Enclosed, warmed tank with a mechanical, hydraulic, or gas mixing system;
- Covered anaerobic lagoon digester — Wrapped with a flexible cover, with methane recuperated and piped to the combustion device;
- Dry digester — Upright, silo-style devices made of concrete and steel with rigid cover.
- Plug-flow digester — Stretched, narrow concrete tank with a shield (rigid or flexible). The tank is fabricated partially below grade to curb the demand for supplemental heat;
The development of biogas has been uneven over the world since it depends not only on the availability of feedstocks but also on regulations that encourage its production and use. Ninety per cent of the world’s output is produced in Europe, China, and the United States [4]. Likewise, Germany, Sweden, the U.K., the U.S., and Switzerland were the largest biogas producers as vehicle fuel in recent years.
The vast bulk of biogas is presently produced in Europe. Here, power (62%) and heating (27%) are the two main uses of biogas. About 11% of the energy produced from biogas is contributed by biomethane used directly in the transportation sector or injected into the grid for use in buildings (mostly for heating or cooking). In Sweden, the Netherlands, and Germany, biogas is converted to biomethane in significant amounts, while in terms of absolute production, Germany generates 33 PJ or 788 ktoe of biomethane [5].
About 70% of the installed biogas capacity in China comprises household-scale digesters in rural areas designed to enhance access to clean cooking fuels. Additionally, the National Development and Reform Commission supported the use of biomethane in the transportation sector by publishing a guidance document on biogas industrialisation and upgrading to biomethane in late 2019.
Nearly 90% of the biogas in the United States has historically been produced by landfill gas collection. Since domestic livestock markets account for almost one-third of the U.S.’s methane emissions, agricultural waste is a topic that is receiving more attention. Due to support from the state and the federal government, this nation also sets the standard for using biomethane in the transportation sector globally.
The majority of the remaining supply comes from developing Asian nations, particularly Thailand and India. The former uses waste streams from its pig farms, biofuel industry, and cassava starch sector to make biogas. Over the coming years, India plans to build over 5,000 new compressed biogas facilities. Both Brazil and Argentina have backed biogas through auctions; Brazil has seen landfills produce most of the fuel, but vinasse, a by-product of the ethanol industry, offers potential as well. The lack of information makes drawing a precise picture of current biogas consumption in Africa more challenging.
3 Biomass Combustion Equipment
3.1 Boilers
Detail on various boiler designs, their application, and equipment offered by specific vendors follows.
Boilers may be categorised into two braod classes: fire-tube boilers and water-tube boilers. In the fire-tube design, the hot gas travels through steel tubes and through a water jacket; in the water-tube design, the heated water transiting steel tubes heated externally by the gases from combustion. In order to sustain pressure and temperature strains, larger shell diameters necessitate thicker plates. Because of its smaller component sizes and ability to accommodate expansion, the steel water-tube boiler is more advisable for large capacities and high pressure.
Besides the aforementioned, boiler designations can be made in another manner: they can be package or field-erected boilers. A package boiler can generally be shipped by standard transportation methods such as a flatbed truck or railcar. The central boiler components are in one body and can be lifted onto a rudimentary foundation and piped into an existing system. The field-erected unit often requires single welding of boiler tubes to the sheets or steam drum and the fabrication of a full steel framework. The parts of a field-erected boiler are entirely built up on site, whereas the package boiler is roughly finished when it leaves the factory.
Finally, the way that combustion occurs can distinguish different boilers. Pile burners, cyclone, and suspension burners are examples of common furnace/burner designs, and fluidised-bed combustors. Boilers incorporating a pile-burning design are employed in applications in which the envisioned wood fuel has a high moisture content (up to 65%), as found in green mill residues, whole green tree chips, and bark. Size control of the fuel is not critical. Grates support the fuel as it dries and the volatiles are pushed out. Several things are accomplished by the air that rises through the grates (underfire air); providing oxygen for combustion, cooling the grates, promoting turbulence in the fuel bed, and contributing to drying the fuel.
Similar to other significant purchases of this nature, buyers of boilers should visit a functioning installation before making their own. The majority of boiler manufacturers are happy to set up such a visit. Purchasing the first of anything carries risks, thus the consumer should be clear about their expectations for “tried and true” products or are on an “R&D” path and willing to take on more risk [6].
3.2 Hot Oil Systems
For relatively high-temperature heating applications, thermal oil is most frequently employed. Due to the necessity for high process temperatures as well as the natural availability of biomass from the plant’s own waste streams, these are most frequently encountered in the manufacturing of lumber and boards.
Steam or thermal oil may provide process heat, somehow interchangeably, up to certain temperature ranges. However, as process temperature requirements go above approximately 450°F, steam pressures become very high. Consequently, steam system components become more expensive, and process equipment design requirements to safely operate steam systems at such high pressures become complex. Due to the lower pressures and the fact that thermal oil systems operate in a single phase, piping and process components may be designed to simpler codes.
A simplified thermal oil system is shown in Figure 4. Two kinds of heat transfer fluids are commonly available: organic-based mineral oils and synthetic heat transfer fluids. Of these, the most common by far are petroleum-based organic mineral oils due to their ready availability, low toxicity, easy handling, lower viscosity (at low temperatures), and much lower cost when compared to synthetic fluids.
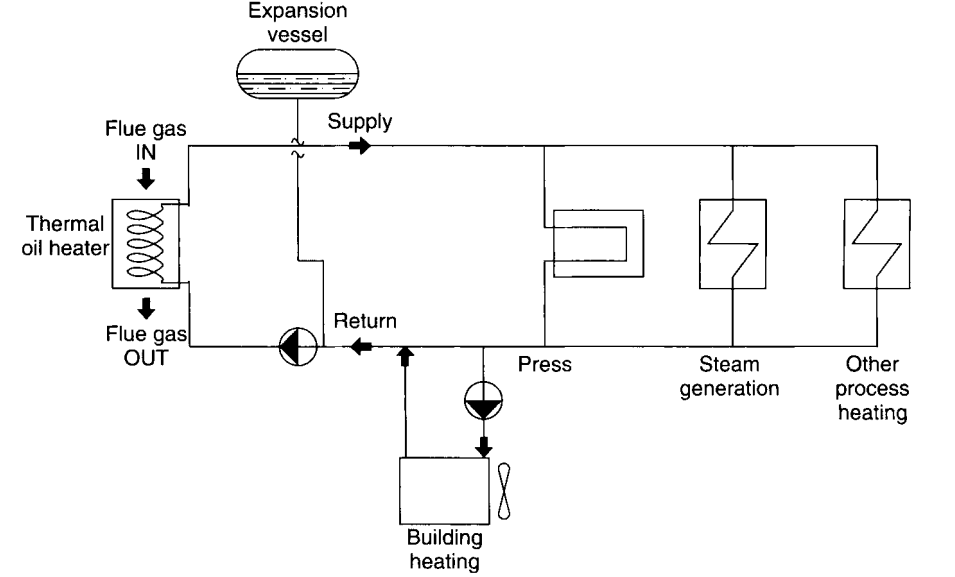
Figure 4. A simplified thermal oil system. Source: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470925324
Thermal oil heaters come in several different arrangements, depending on the size of the heater and the specific application. The simplest and perhaps lowest-cost design in biomass applications consists of the simple helical coil. Steel pipe is rolled into a helical coil pattern, with thermal oil on the inside and hot combustion products flowing through the centre of the coil. Heat is transferred from the gases principally by radiation but also partially by convection.
Another common design is the “convective” tube bundle design. In this design, the heater tubes are arranged in a serpentine flow pattern, with the thermal oil flowing inside parallel banks of tubes oriented perpendicular to the gas flow. The thermal oil flows down one tube, then the flow is turned around via a 180° bend and returns down the next row of tubes. This serpentine flow pattern continues for several passes until the oil finally exits the convective tube bundle.
Primary Loops
Thermal oil circulation consists of basically two main types of circulation loops. The primary loop’s purpose is to maintain a safe rate of flow of the thermal fluid through the thermal oil heater, as well as to supply a sufficient flow of hot thermal fluid to all of the secondary loop heat users. For any thermal oil system, the design of this loop is critically important to the safety, reliability, and life of the thermal oil heater and associated system components.
In a properly designed system, the functions of the primary circulation system are to i) provide an adequate primary flow of thermal fluid through the heater, ii) take care of the thermal expansion of the fluid, iii) provide means for deaerating the fluid, and iv) provide the secondary loop with adequate thermal oil and, hence, heat energy supply.
Secondary loops
Generally, heat demands in thermal oil systems are supplied by the secondary loop circulation system if the primary pump pressure is not sufficient to supply a single user or if multiple users are tied to a primary loop. Typical secondary loops consist of a circulating pump, shutoff valves, and one or more control valves that control the amount of hot oil supply that is fed into the user.
Unlike primary loops, redundant pumps are generally not used unless the process is very critical since there is no heat-generating equipment contained in the secondary loop that could result in oil degradation, equipment damage, or unsafe conditions.
3.3 Components of Hot Gas Generation
The components of a hot gas generation system generally consist of a furnace/combustion system, a post-combustion/secondary chamber, and a hot gas control system.
The furnace or combustor is generally of the type described previously for biomass combustion. A well-designed system provides a good post or “secondary” combustion chamber to complete combustion and reduce the spark carryover into the heating process. Additional components to reduce spark and ash carryover may be included for critical hot gas processes if the downstream processes or products could be affected.
Most hot gas systems must provide hot has at a constant regulated temperature. As biomass fuel quality can vary significantly, and often the hot gas temperature requirement is lower than that of the combustion process, gas temperature regulation is generally needed. This is typically done using an air dilution or blending system, where dilution air is added to the exhaust stream of the combustor in the proper regulated amounts needed to maintain a constant hot gas supply temperature.
When different process needs are required, thermal oil, steam, and hot gas systems are often combined into a single system. In board plants, it is common practice for biomass systems to provide thermal oil process heating for various process needs, such as pressing board, steam generation, building heating, and other uses, while simultaneously providing hot gas energy for the drying of wood flakes before the pressing of board. In some cases, this may also be combined with direct or indirect steam generation for either process needs or power cogeneration.

Figure 5. The two main types of biomass gasifiers. Source: https://www.teriin.org/technology/biomass-gasifier-for-thermal-and-power-applications
4 Anaerobic Digestion Components
4.1 Planning and Design Factors
Anaerobic digestion is a process used in many different technical applications for the treatment of organic solid waste for gas production and waste stabilisation [7]. Technologies have been developed for the treatment of many kinds of organic matter, such as wastewater sludge, municipal organic solid waste, high-strength industrial wastewater, manure, and mixtures of different types of organic waste.
Today, the know-how is successfully used within all these areas. The technology range from sophisticated, highly engineered large-capacity plants to simple single-family plants operated by the owner. The field is young and immature, as the first full-scale commercial plant came into operation in 1986.
Many companies have worked in the field, but no single company or technology has a significant market share. From the start in the mid1980s in Western Europe, it took more than ten years to reach 70 plants. The plants ranged from a few hundred tonnes/year up to more than 100 000 t/year. The International Energy Agency once reported that more than 100 plants are in commercial operation, and half of all plants are in Germany. Also, Switzerland, Sweden, and Austria have a significant number of plants.
Due to the immature technology and the many different technologies and companies involved in the design and construction of a relatively small number of plants, no generally accepted design rules for the other parts of anaerobic digestion plants are available. Each company involved seems to use its own design criteria and rules. Only for the digestion process itself, there seems to be some kind of agreement on a proper organic loading rate and a minimum solid retention time depending on the selected process. Table 1 states the typical design values for municipal organic solid waste [8].

Table 1. Design considerations
4.2 Technology Selection
Reception of Waste
The reception of waste is a very simple but also vital part of the whole system. Great variations in waste delivery have to be properly organised in order to be able to receive the waste and to feed the treatment system relatively constant as required by the biological processes. Further, the possibility of identifying unwanted waste types is essential.
Many plants have had great trouble with the poor design of this part of the system because too little attention is put on the handling of all the different kinds of waste that may arrive at the plant. Available waste reception technologies include a weighbridge, tipping place, and bunker.
Pretreatment
The pretreatment must be able to remove all kinds of misplaced items in the arriving waste. It is not enough to select the pretreatment based on only an evaluation of the sorting criteria and collection system alone. Further, economic considerations have a significant impact as any separation leads to the creation of a reject stream that can be very costly to eliminate and which contains organic material together with the material that has been separated. Different separation systems have very different separation efficiencies, and the need and price for separating missortings must be balanced with the loss of organic material.
Typically, several steps are necessary to get sufficient reduction of missortings. Usually, a grinder or shredder is used as the first step in the pretreatment process in order to open the collection bags and break down the big items. Next, pretreatment systems typically focus on three different foreign items. First, any magnetic material is removed in a magnetic separator. Then any heavy, rigid material that can wear down the machinery in the plants is separated by gravity.
Further, plastic or other lightweight material, not considered biomass or part of the residue, is sorted out by gravity or some kind of sieving. Finally, hygienisation is often introduced as part of the pretreatment, where the biomass is heated to destroy any pathogens present in the waste before anaerobic digestion.
The various technologies are bag openers/size reduction, magnetic separation, separation by gravity in a pulper, separation by size using sieves, separation by size and gravity on a disk screen, separation in presses, hygienisation, and separation by hand.
Digestion
Anaerobic digestion shall convert the waste into biogas and digest. The digestion system is comprised of the following functions:
- Storage and feeding system.
- Preheating of the biomass.
- Mixing of new biomass and active microorganisms.
- Gas collection system.
- Separation of solid and liquid digest.
In all cases, the technology involves storage and feeding systems in order to facilitate controlled feeding of the system in spite of the variation in waste delivery. The feeding system has to secure a proper feeding according to the selected loading and feeding strategy (batch, continuous or semicontinuous operation). In some cases, the storage system is used for mixing the incoming waste with the active biomass.
The waste always needs preheating to obtain the selected process temperature. Normally, a heat exchanger will suffice to transfer heat from the outgoing digest to the incoming waste. In the case of thermophilic digestion, more heat may be needed, and excess heat from gas motors or combustion of gas is used for the purpose.

Figure 6. Reactors for anaerobic digestion at Deer Island Wastewater Treatment Plant, Massachusetts. Source: “Anaerobic Digesters” by Massachusetts Clean Energy Center is licensed under CC BY-NC 2.0.
The digestion takes place in one or more reactors depending on the selected process scheme. In wet processes, the reactions take place in a mixed reactor due to the fluid character of the biomass. New waste is introduced into the reactor and easily mixed with the active biomass by gentle mixing either mechanically or by recirculation of gas inside the reactor. In dry processes, where the biomass is a thick slurry, the flow in the reactor is more or less plug flow and mixing of waste, and active biomass takes place prior to introduction in the reactor. In phased processes, where the methanogenic biomass is placed as a biofilm on carrier material, the contact between biomass and the organic material is usually ensured by the system’s hydraulic turbulence without a particular need for mixing.
Biogas produced in the digestion process has to be collected. In wet systems, the gas is simply released at the surface of the reactor content and can be withdrawn. The mixing in the reactor is sufficient to get a good separation of gas from the waste. In dry systems, gas can be entrapped in the biomass, and a very gentle mixing is needed to get the gas released to the top of the reactor.
After digestion, separation of solid and liquid digestate is normally needed. In wet systems part of the wet digest is recirculated for dilution of the incoming waste and part is withdrawn as liquid digestate. In dry systems part of the residue is recirculated as inoculum for new waste, whereas the rest proceeds to the posttreatment. Normally a minor fraction of wet digestate is separated in order to have a good product for the following posttreatment of the solid digestate.
Gas Handling
The gas storage and treatment system collects and prepares the produced biogas for the final gas utilisation. The need for gas treatment depends greatly on the final utilisation of the gas. The gas from the digestion process is normally saturated with water and has a methane content of about 64 %. Further, it contains carbon dioxide and has minor hydrogen sulphide and ammonium content. The various technologies are briefly described below.
The need for gas storage highly depends on the expected use of the gas. Sometimes no storage is needed because the gas is delivered to a power plant or directly incinerated for heat and power production. In other cases a, storage is required in order to get a smooth operation of the gas handling facilities. Finally, payment of electricity produced from biogas may vary during the day, meaning that electricity is economically advantageous only during short periods of the day.
Four main applications with increasing demand for gas treatment are common. In all cases, a flare is used as a backup if the present gas utilisation is out of order:
- Heat production: The most straightforward use of biogas is to incinerate the gas directly in a boiler for internal use of heat at the plant or in a local district heating network. Such utilisation is possible at small plants where the cost for further treatment is too high to be attractive under the actual economic conditions.
- Power and heat production: Power and heat production is the most common application for biogas utilisation. The requirement for pretreatment is moderate and consists of the removal of water and hydrogen sulphide. Then the gas can be utilised in a standard gas engine.
- Vehicle fuel production: Upgrading gas to vehicle fuel requires higher methane content than obtained in the digestion process. Besides water and hydrogen sulphide, most of the carbon dioxide must be removed to reach a methane content above 95 %. Different commercial approaches exist for such upgrading.
- Upgrading biogas to natural gas quality: Upgrading biogas to natural gas quality requires even higher methane content than upgrading to vehicle fuel. Different technologies are under development, but they are expensive and only used in a few cases. The upgrading is attractive in areas where a natural gas network exists, as it is pretty simple to have a smooth and safe delivery of the gas. However, at the moment, the process has to be highly subsidised.
5 References EF
[1] FAO: Bioenergy and Biofuels Factsheet.
[2] Global Potential of Sustainable Biomass for Energy
[5] Kampman, B., Leguijt, C., Scholten, T., Tallat-Kelpsaite, J., Brückmann, R., Maroulis, G., … & Elbersen, B. (2017). Optimal use of biogas from waste streams: an assessment of the potential of biogas from digestion in the EU beyond 2020.
[6] Brown, M. L., Bulpitt, W. S., Walsh Jr, J. L., & McGowan, T. F. (2011). Biomass and alternate fuel systems: an engineering and economic guide.
[7] https://www.epa.gov/agstar/how-does-anaerobic-digestion-work
[8] Christensen, T. (Ed.). (2011). Solid waste technology and management. John Wiley & Sons.

 To all knowledge
To all knowledge