Corrosion Protection of Offshore Facilities: Corrosion is a naturally occurring phenomenon commonly defined as the deterioration of a substance (usually a metal) or its properties because of a reaction with its environment. Like other natural hazards, corrosion can cause dangerous and expensive damage to everything from offshore structures and pipelines to ship hulls and superstructures, inner-hull tanks in fuel tankers, underwater pipes, etc. According to the current NACE ‘Cost of Corrosion’ study, the direct cost of metallic corrosion is $276 billion on an annual basis in the US (1). Breaking down this number, however, displays the magnitude of the role coatings plays in preventing corrosion. There are a wide variety of technologies designed to minimise corrosion, from coatings to cathodic protection, advanced materials design, chemical inhibition, etc. The Cost of Corrosion study found that the cost of these technologies’ totals over $121 billion per year. Of that, however, 108 billion dollars is spent is in the coatings service sector. This means that there is constant pressure on the coatings industry to stay at the forefront of corrosion prevention technologies, providing cost-effective solutions in the harshest of conditions. Advances in coating technology can offer significant cost saving and performance advantages if developed, specified, applied and maintained correctly.
Further to this, the protective coating (PC) industry is entering a new era with legislation being introduced internationally aimed at reducing the emission of volatile organic compounds (VOCs) from coatings. To meet the new VOC standards, traditionally effective and trusted corrosion inhibiting coatings can no longer be used, and high-performance replacements need to be found and necessitate significant changes in the raw materials and formulation of anticorrosive coatings which have traditionally contained a relatively large amount of organic solvents. To comply with new legislation, the industry as a whole is moving towards to use of water-based coatings, powder coatings and coating systems with high solid contents (2). However, it remains a challenge to completely replace traditional solvent-based coatings, particularly in harsh conditions. Critical to the successful formation of coatings for these applications in marine and offshore sectors is a sound understanding of the environment in which it will be applied, the interaction of the coating with the environment, the interaction of the components of the coating with one another, and the potential failure mechanisms for a coating system in a harsh environment.
To better understand the impact of each of these areas, this article includes a general classification of the most common types of corrosion and their failure mechanisms, a description of the expected environments an anticorrosive coating is likely to encounter, an overview of the different families of anticorrosive coatings available including their primary components and working mechanisms, and new coating systems appearing on the market.
The basics Corrosion Protection of Offshore Facilities – what is corrosion and why does it happen?
It’s common for metallic structural components to experience some form of corrosion regardless of what type of material is used. Corrosion-resistant coatings are designed to increase the lifespan of a part while reducing on-going maintenance and replacement costs. However, selection of the most appropriate coating for an application, it’s necessary to identify the type of corrosion a structure, part or piece of equipment is prone to. Based on how a part is designed to be used and the conditions it’s exposed to, the kind of corrosion and the required prevention mechanism differs greatly.
While there is a large number of different types of corrosion, there are eight general types of corrosion as defined by Fontana and Greene (1967), all of which are relevant to offshore infrastructure. They can be classified by the form in which each manifests itself, and can typically be identified by the naked eye. Valuable information for the solution of a corrosion problem can typically be obtained through careful observation of test specimens, failed equipment, or systems currently in the field. Note also that sometimes observed corrosion is not attributable to a single cause, but can sometimes be the result of several interrelated causes.
The first and most common type of corrosion is a uniform attack, characterized by a chemical or electrochemical reaction which proceeds uniformly over the entire exposed surface. Failure usually results from the thinning of the metal over time. The uniform attack represents the greatest destruction of metal on a tonnage basis, but it is also one of the more easily addressed forms of corrosion. It is also possible to accurately estimate the rate of corrosion through simple comparative testing.
Another commonly encountered form of corrosion is galvanic corrosion. Any time two dissimilar metals are coupled in a solution a potential difference exists, making one of the metals a cathode, and the other the anode. This drives the flow of electrons between the two metals. The net result is that corrosion of the anode is accelerated, while the cathode corrodes at a reduced rate (when compared with their normal corrosion rates). This phenomenon forms the basis of cathodic protection systems.
A slightly less obvious form of corrosion is crevice corrosion, synonymous with intense localized corrosion occurring frequently within cracks and crevices and other shielded areas on metal surfaces. This type of attack is usually associated with small volumes of the stagnant solution caused by holes, gasket surfaces, lap joints, surface deposits, and crevices under bolt and rivet heads.
Pitting is another form of localized attack that typically results in holes or cavities in the metal, typically small in size. Pits can be isolated or grouped close together giving the appearance of a rough surface. Pitting is one of the most destructive and insidious forms of corrosion. It can cause material failure with only a small per cent weight loss of the entire structure due to perforation. Due to pits being small and easily obscured, they are also extremely difficult to detect or measure the extent of pitting quantitatively. Pits are also difficult to analyse for under test conditions, making the rate of corrosion particularly difficult to forecast, further exacerbating the danger pits present.
A far less common form of corrosion is intergranular corrosion, the localized attack along the grain boundaries, or immediately adjacent to grain boundaries, while not having any significant effect on the grains. Since it only takes place at the grain boundaries of metals, in most applications it is largely inconsequential. However, under certain conditions, grain interfaces are very reactive and intergranular corrosion results.
A similar type of corrosion is selective leaching, also known as dealloying, which is the selective removal of one component from an alloy by corrosion processes. A common example is the selective removal of zinc in brass alloys but similarly occurs in other alloy systems from which aluminium, iron, cobalt, chromium or other elements are removed.
Erosion corrosion is the acceleration of the rate of corrosion due to the flow of a corrosive fluid over a metal surface. The relative motion of the metal surface and corrosive fluid causes turbulence which can result in rapidly increasing erosion rates. Typically characterized the appearance of surface grooves, gullies, waves, rounded holes, and valleys in a directional pattern, erosion-corrosion is also sometimes caused by poorly finished surfaces or poor workmanship. In many cases, material failure due to erosion-corrosion is unexpected and happens in a relatively short space of time, usually because the predicted corrosion was evaluated under static conditions and flow-driven erosion effects were not considered.
The final form of corrosion is known as stress corrosion cracking (SCC) which is defined as the growth of cracks due to the simultaneous application of stress and exposure of the surface to a specific corrosive medium, leading to unexpected catastrophic failure of ductile metals. Cold forming, welding, heat treatment, machining and grinding can all introduce residual stresses, the magnitude and importance of which are often underestimated. The SCC sites on the metal surfaces may not be visible by visual inspection, while metal parts are being filled with microscopic cracks. These invisible cracks progress rapidly and lead the component and structures to catastrophic failures (3).
While understanding the different forms of corrosion is important in preventing them, equally important is knowing the environment in which the infrastructure will reside.
Corrosion Protection of Offshore Facilities: The Corrosive Environment
High-performance anticorrosive coatings are applied to areas within a variety of environments, introducing several unique conditions the coating needs to be resistant against. Some of these include constant or partial immersion in water or soil, atmospheric pollution in industrial settings, as well as ultraviolet radiation in outdoor cases. The variety of environmental factors as well as the exposure time to these factors impacts heavily on the coating requirements, and therefore formulation, making knowledge of the environment paramount to the successful coating selection.
To better understand the corrosive conditions a coating is likely to encounter, ISO 12944 ‘‘Coatings and varnishes—Corrosion protection of steel structures by protective coating systems’’ presents a classification system that divides environments into three broad types of exposure: immersion, atmospheric and splash zone.
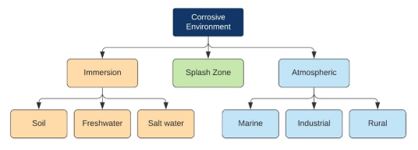
Figure 1: Classification of corrosive environments (2)
Immersion applies to any structures immersed in water or soil. In these cases, the severity of the environment is extremely specific because of the multitude of factors that can play a role in overall corrosivity. Salinity, pH, presence of dissolved gases and temperature, amongst others, are all known to contribute to creating a corrosive environment. Due to the wide variety of conditions that can be encountered, they are classified by ISO 12944 into 3 categories as per Table 1.

Table 1: Immersion Categories for Soil and Water (4)
The corrosivity of soil on buried structures and coatings is mainly determined by the type of soil, pH, humidity, oxygen content, and presence of bacteria and salts. The factors for the two aqueous environments also differ significantly from one another. The corrosivity of a freshwater environment is largely defined by the type and concentration of dissolved salts and oxygen (5), along with the expected flow. Higher flow regions introduce a mechanical aspect, with coatings needing to be resistant against erosion and impact from suspended solids. In comparison, saltwater has a far higher concentration of salts, particularly chloride, which are extremely aggressive towards both coatings and metals alike. Also requiring consideration for both salt and freshwater is the potential for biofouling (2), and the effect this can have on the performance of the coating.
Atmospheric environments can vary greatly, and have alternating conditions which are controlled by heat, light, ultraviolet radiation, relative humidity, along with more constant factors like chemical concentrations, pollution levels proximity to moisture and distance to the sea. The relative combination of these factors creates a wide range in the corrosivity of the environment, which are defined by 6 corrosivity categories ranging from C1 to C5-I/M based on expected mass and thickness loss for steel and zinc (Table 2). These classifications are somewhat subjective, and a location could fall inside a number of the categories depending on specific conditions. However, they do provide a simple frame of reference when areas are being evaluated for coating application in mind.

Table 2: ISO 12944-2 Classification of Corrosive Environments (4)
The need to create these categories is driven by the different environments having different contributing factors to corrosion, and subsequently different corrosion mechanisms. For coatings to be suitable for a specific area, they need to be resistant to the dominant corrosion mechanism in the area. For example, rural environments typically have low levels of corrosivity when compared with heavy industrial or marine/offshore environments. Industrial environments are typically classified by high levels of airborne particles, including but not limited to soot, sand and sulphate salts. Also of concern are higher than average levels of gases such as sulphur dioxide, which when combined with rain can create an extremely acid environment. Marine and offshore environments are characterized by having a very high content of chloride ions, which are very aggressive towards metals and may cause pitting corrosion (2). Therefore, these three environments have very different requirements when evaluating an anticorrosive coating system, and must be considered separately.
The final environment, and also of particular relevance to offshore structures, is the ‘splash zone’, characterised by on-going intermittent wetting and drying due to waves, wind or tides. Corrosion rates in this zone are typically the highest of all the zones, driven by several factors. Firstly, the constant splashing aerates the water, increasing the availability of dissolved oxygen for the electrochemical reactions. Secondly, the cyclic wetting and drying increase the concentration of chloride ions on the surface, again accelerating the corrosion (5). Finally, and of particular concern for coatings, is the exposure to ultraviolet radiation and the abrasive effects of seawater containing a sand and other solid debris contributing a mechanical component to the coatings’ deterioration in this environment.
Corrosion Protection of Offshore Facilities: Anticorrosive coatings
Protective coatings represent one of the simplest ways to reduce corrosion, largely by limiting the exposure of the metal to a corrosive environment. By adhering to the substrate, the coating prevents the corrosive elements from coming into direct contact with the metal. Protective coatings can be sprayed, rolled or brushed on, plated on, or applied using hand tools. Besides corrosion resistance, protective coatings can also be fundamental in allowing a substrate to withstand issues such as wear and exposure to water. They can also improve the asset’s aesthetic appearance.
Anticorrosive coatings vary depending on the type of metal involved, the area of application, the predicted exposure the metal will experience during its lifespan and the kind of corrosion prevention needed. For example, the prevention of galvanic corrosion in iron and steel alloys is typically achieved through the use of coatings made from zinc and aluminium. Large components, such as bridges and energy windmills, are often treated with zinc and aluminium corrosion-resistant coatings because they have been proven to provide reliable long-term corrosion prevention.
In addition to zinc, and aluminium coatings, often nickel-chromium and cobalt-chromium are used as corrosive coatings because of their low level of porosity. They are extremely moisture resistant and therefore help inhibit the development of rust and the eventual deterioration of the metal. Oxide ceramics and ceramic metal mixes are examples of coatings that are strongly worn resistant, in addition to being corrosion resistant.
A typical anticorrosive coating system for marine and offshore environments (C5-M/CX) usually consists of a primer, one or several intermediate coats, and a topcoat (6). The primer has two main functions, the first of which is to ensure good adhesion of the coating system to the substrate. Without excellent adhesion, no amount of anticorrosive properties will have any effect on protecting the metal, and failure of the coating system is almost guaranteed. Secondly, the primer also protects from corrosion. Primers typically contain anticorrosive additives such as metallic zinc or specialist pigments to provide this functionality. The intermediate coats are generally applied to build up the thickness of the coating system and impede diffusion from the surface to the substrate it is protecting. Increasing the thickness creates a longer diffusion path, as well as providing mechanical strength to the system by distributing stresses. Additionally, the intermediate coat must also ensure good adhesion between the primer and the topcoat. Finally, the topcoat is what is visible and exposed to the external environment. It is designed to provide the surface with the required aesthetics (colour and gloss), resistance to weathering, mechanical impacts and abrasion, as well as excellent resistance to ultraviolet radiation.
There are a wide number of factors that contribute to an anticorrosive coating, and all need to be assessed to ensure the successful performance of the coating system. Figure 2 illustrates some of the most significant factors that need to be considered.
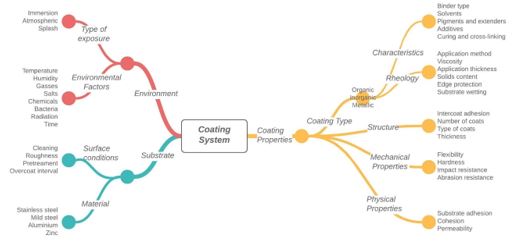
Figure 2: Factors affecting coating system performance (modified from (2))
Typically, anticorrosive systems are classified by how they protect with the substrate, with three major classes of protection existing: barrier protection, inhibitive effect (also known as passivation) and galvanic effect (or sacrificial protection). Barrier protection is simply where the coating system acts as a layer with very low permeability for liquids, gases and ions, the most important of these being organic coatings. Barrier coatings are typically specified on immersed structures and applied as a conventional multilayer system (primer, undercoat and topcoat). They typically contain inert pigments such as coated titanium dioxide or iron oxide, and very low pigment volume concentrations (PVC) driven by higher polymer content making the permeability to water and gas very low (2). Also requiring consideration is the degree of crosslinking of the polymer, which creates a more macroscopically intact film resistant to diffusion. However, any polymer film will remain penetrable to some extent by gases and electrolytes, due to diffusion and migration. Increasing the film thickness is one possible solution to increase the diffusion length (6). It is typically recommended that for the optimal anticorrosive performance of barrier systems, the film thickness is increased through multiple thin coats rather than the application of a single thicker coat. However, this is not always practicable due to time and cost implications.
When examining the working mechanism of barrier coatings, it is important to note that while acting as a barrier to water and oxygen is important, the major contributor to their anticorrosive functionality is their ionic impermeability, which ensures that any moisture at the coating-substrate interface has a very high electrical resistance. Thus, the conductivity of the electrolyte solution is so low that the transfer of corrosion current between the anode and cathode is minimized, preventing corrosion from taking place on the surface of the substrate (2). These coating systems are typically augmented by cathodic protection, and in the majority of offshore structures, using a sacrificial anode usually in the form of an aluminium alloy, zinc, or magnesium.
Sacrificial coating systems are designed around the principle of galvanic protection to minimise corrosion of the substrate. In this case, the coating is formulated using more electrochemically active metals such as metallic zinc powder for protecting steel structures. This form of protection however requires the coating to be in direct contact with the substrate, thus making this type of coating only suitable as a primer. These coatings are sometimes called an anodic coating because the zinc behaves as a sacrificial anode for the steel. The anticorrosive performance is entirely is dependent on the transfer of galvanic current by the zinc, but as long as the system remains conductive, and there is sufficient zinc to act as an anode, the metal will be galvanically protected. Due to the requirement for metallic contact between the substrate and the zinc, these coating systems typically have a very high solids content, with up to 95 wt% being metallic zinc. Due to this high zinc content, the coatings have a very little polymeric binder, making them vulnerable in terms of strength, adhesion, flexibility and impact resistance. They, therefore, need to be applied with care in appropriate locations.
The final category of coatings works by passivating the surface of the substrate, resulting in a build-up of insoluble metallic complexes which act as a barrier to any corrosive elements. These coatings also rely on direct contact with the substrate, and as such can only be applied as primers. They are also typically recommended for atmospheric environments, and not for immersion in water or soil.
Main components of an anticorrosive coating
Designing an anticorrosive coating requires a sound knowledge of each of the components in the formulation, the function of each of these components, the intended application of the coating, the required level of performance and lifespan, and economic evaluation to target a price point for the finished formulation. Coatings can be formulated from an enormous variety of chemicals or a combination of different chemicals, and there are usually several ways to achieve the same outcome. To better understand the roles of different components in the formulation, they are broken down into 5 main categories:
- Pigments – Insoluble particles which have two main roles in a coating: to provide colouration, and to impart specialist performance e.g. corrosion resistance, special effects, etc.
- Binders – Binders are the ‘glue’ holding a coating together, polymeric film formers that enable bonding between the pigments and between the surface and the pigments (7). Examples include alkyds, acrylics, epoxies and polyurethanes.
- Extenders – normally naturally occurring minerals added to coatings to increase solids content without increasing costs, provide brightness and strength, and wet state rheology control (8). They do not typically contribute to the anticorrosive performance. Examples include calcium carbonate, talc and kaolin.
- Additives – covers a vast variety of chemicals typically added in small concentration to achieve a certain outcome, e.g. surfactants, wetting agents, biocidal agents, antifoaming agents, anti-settling agents, rheology modifiers, UV light absorbers, curing agents, film-forming solvents,
- Solvents – A volatile liquid, consisting of one or more components, and can dissolve binders and other constituents without the need for a chemical reaction (7). Most solvents are volatile under normal application conditions and make paint processing suitable for the application.
The pigment, binder and solvent all play significant roles in anticorrosive coatings, and as such will be discussed separately. Additives represent a huge variety of specialist chemicals designed for specific applications, and discussion of the full gamut of additives falls outside the scope of this article.
Corrosion Protection of Offshore Facilities: Anticorrosive pigments for offshore applications
Any discussion of pigments in coatings would be remiss if it didn’t include a definition of the pigment volume concentration (PVC), an important value to be considered when formulating. PVC represents the proportion of pigments and extenders in the dry paint film (Equation 1).

The lower the PVC, the higher the quality of the paint as the pigments and extenders represent a lower proportion of the dry film volume, with binder solids taking up a higher proportion. The PVC should always be considered alongside the critical PVC (CPVC), which represents the point at which there is sufficient binder in the system to entirely coat the discrete pigment and extender particles (9). If the PVC is lower than the CPVC, then there is sufficient binder to wet and fill the voids between particles, and the final film is uniform. This is important for corrosion protection as the coating is a barrier to water, oxygen and other corrosive species and thus anticorrosive paints are typically formulated to be below CPVC. Alternatively, when the PVC is higher than the CPVC, the presence of air in the voids between particles caused by the insufficient binder dramatically changes the properties of a coating (2). CPVC can be determined experimentally or estimated using the oil absorption of the pigments and extenders.
Pigments which play a role in imparting anticorrosive properties to a coating can be classified in three broad categories: barrier, sacrificial and inhibitive. Barrier pigments typically act physically, using a flat, flake-like lamellar structure with a high aspect ratio to lengthen the diffusion path for corrosive species through the dried paint film. This has the additional benefit of providing internal reinforcement to the paint film, increasing mechanical strength and inter-coat adhesion while reducing UV absorption. Of these pigments, the most widespread is micaceous iron oxide (MIO), a crystalline form of iron oxide (α-Fe2O3). Named because it fractures into thin flakes when dispersed, giving a final appearance very similar to that of mica (6). To achieve optimal barrier properties, coatings using barrier pigments are typically formulated to a PVC of between 25 and 40%, well below CPVC.
Other commonly found barrier pigments include lamellar forms of aluminium, both leafing and non-leafing varieties. Leafing varieties are typically surface treated during manufacturing to lower surface tension and cause the pigment to ‘float’ to the surface of the coating (2). For this reason, they typically require overcoating to protect the metallic finish from unwanted corrosion processes and pigment abrasion (6). Non-leafing aluminium pigments orientate randomly and homogeneously throughout the coating, forming extremely effective barrier coatings. Glass flakes are also employed as barrier pigments due to their inert properties, but their relatively large size (100-400µm) necessitates their application only in very thick coatings. Glass flake technology has been a mainstay of the oil and gas (O&G) industry for many years, particularly for highly aggressive splash zone areas. Particularly when coupled with other functional pigment systems and advance polymeric binders, glass flakes can deliver effective long-term protection of tanks, vessels and pipelines, as well as immersed or atmospheric structural steelwork.
Sacrificial pigments can be any metallic pigment which is more electrochemically active than the substrate. In practice, it is largely only metallic zinc particles which are used in this application, however, magnesium is becoming increasingly used for the protection of aluminium. Zinc in the coating protects a ferrous substrate by acting as a sacrificial anode which corrodes in favour of the ferrous cathode. Critical to the performance of sacrificial pigments is particle size, with smaller particles offering a far greater surface area for reaction. Also of importance is the particle shape and the width of the particle size distribution. Non-uniform shape and a broad distribution offer improved packing compared with spherical particles of a single size. Improved packing reduces porosity and permeability, as well as increasing the number of electrical contact points which improves the ability of the coating to conduct the galvanic current away from the substrate. Coatings containing zinc dust are widely used in both organic and inorganic matrices for the protection of structural steel, offshore and marine coatings and underwater steel constructions (6).
Corrosion inhibiting pigments (also known as active pigments) are the most complex of the corrosion protection pigments, and function by disrupting the corrosion reaction typically through a competing reaction, but the exact mechanism or reaction depends on the specific pigment type. Coatings utilizing inhibitive pigments release soluble species from the pigment into the electrolyte once it has saturated the coating. These species then inhibit corrosion in a process called passivation by facilitating the growth of insoluble protective layers on the substrate surface. Passivation refers to the formation of thin oxide layers, and the metallic component losing its reactivity (10). Some of the most popular inhibitive pigments are either lead-containing or chromates, both of which are widely used and known to be extremely effective corrosion inhibiting pigments. However, due to the toxicity and known carcinogenic nature of these pigments, and the introduction of legislation to very tightly control their use, manufacturers are actively seeking replacements. Of these, inorganic phosphates, molybdates and silicates are gaining acceptance and seeing increased application in anticorrosive coating systems.
Corrosion Protection of Offshore Facilities: Solvents
Another major component of any paint is the solvent, which is only intended to be in the film temporarily while it dries. However, solvents play several important roles, including dissolving, dispersing or wetting other components, reducing the viscosity to allow for application, and slowing the drying rate to allow for improved film formation. Coatings are normally classed based on the highest concentration solvent, being either water-based, solvent-based, or solvent-free. As stated earlier, there is a major drive to move towards environmentally friendly formulations and to get away from the dependence on solvent-based coatings, particularly in the performance coating sector.
However, this is not without complication. The solubility of the polymeric binders traditionally used in anticorrosive paints is far lower in water than it is in organic solvents, making dissolution of the polymer problematic. New developments in binders suitable for application in water-based anti-corrosive coatings are styrene-butadiene and vinyl acrylic copolymers, as well as chemically-cured epoxy binders. Water-based systems also have several inherent drawbacks when compared with the solvent-based counterparts, the first of which is the need to counteract the occurrence of flash rust. Other considerations include their inability to endure freeze/thaw cycles, any restrictions on atmospheric humidity during application and curing, and susceptibility to wet-state biological attack from bacteria and fungi. All of these are particularly significant for offshore applications, where storage and application conditions cannot be easily controlled. Thus, the benefits of the use of a water-based coating system need to be carefully weighed up against the increased potential for challenges during application and long-term durability concerns.
An interesting category of coating is ‘solvent-‘free’ systems, essentially 100% solid powder-based systems. These are applied electrostatically and then baked, using thermoset or thermoplastic polymers to provide a durable protective finish. This is considered an environmentally friendly alternative to solvent-based coatings, as the formulations contain no solvents. However, these coatings require baking after application to form an impervious layer, requiring additional infrastructure for the application. This drastically limits the size of the items that can be powder coated, meaning limited application for offshore infrastructure. A further issue is a difficulty with maintenance, as powder coatings can’t be overcoated or touched up.
A wide range of organic liquids, such as aromatic and aliphatic hydrocarbons, glycol esters, and alcohols have been applied in traditional solvent-based coatings. However, the correct choice of solvent technology for anticorrosive coatings depends on the required properties about the conditions of the binder of the system, as well as the environment, method of application, curing, pigmentation, and nature of the substrate.
Corrosion Protection of Offshore Facilities: Common binders for anticorrosive coatings
The binder is responsible for several vital characteristics of a coating, including adhesion to the substrate, cohesion within the coating, mechanical strength, flexibility, durability, resistance to abrasion and resistance to permeability. These properties form as the applied coating transitions from the wet state to the dry state, as the individual polymer particles crosslink when curing. Film formation takes place in one of three ways: drying (evaporation of the solvent from the film), chemical reaction, or a combination of both of these (Figure 3).
The first and extremely common family of reaction-cured binders is alkyds. The etymology of the word alkyd is derived from alcohol and a-CID, later changed to alkyd (6). Alkyd-based coatings are characterised by good adhesion, flexibility, resistance, and durability. Alkyd coatings are very often employed as primers, typically with an inhibitive pigment in corrosive environments. The greatest weakness of alkyd coatings, however, is the potential for saponification, where ester linkages in the resin are attacked by alkaline materials to form an alcohol and the salt of a carboxylic acid. Saponification is particularly problematic when alkyd primers are applied on galvanized surfaces, in zinc-rich primers, or when applied to systems with cathodic protection or sacrificial anodes (2).
The next category, polyurethane coatings, are typified by their excellent resistance to weathering and resistance to UV radiation, making them particularly effective as topcoats for offshore applications. They can also be formulated to be somewhat ‘self-healing’ due to the ability of the hydrogen bonds between the urethane linkages to reform after being broken (2). Polyurethanes are available as two-component solvent-based coatings which are the reaction product of isocyanates with compounds containing an active hydrogen atom (amine or hydroxyl group). Water-borne polyurethanes are also available and are typically two-component systems based on separate dispersions of polyols and isocyanates in water. Compared to two-component solvent-borne coatings, water-borne urethane coatings are lacking in chemical and corrosion resistance and not frequently used in anti-corrosive applications (2).
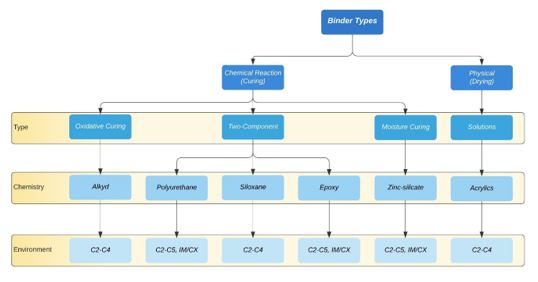
Figure 3: Classification of the binder by curing mechanism and potential application environments (adapted from (2))
Siloxane systems contain polysiloxanes, which are polymers with a silicon-oxygen (Si–O) backbone and are made from monomeric building blocks. In comparison with traditional organic binders, polysiloxane coatings exhibit superior gloss and colour retention but suffer from poor mechanical properties (2). A key strength of silicon-based polymers is their inherent temperature resistant nature. Other strengths include resistance against UV irradiation and oxidation which have motivated attempts to use the chemistry for property improvement of both organic and inorganic systems, including good resistance to certain acids and solvents, high nonvolatile content and good weather ability (6). Further, because the siloxane binder is already oxidised, making it impervious to further oxidation. Siloxanes can also be reacted with epoxy, acrylic or other organic compounds to form hybrid siloxane coatings, which have found widespread usage for industrial application because of the improved adhesion and resistance to moisture brought about by the siloxane (2).
Epoxy resins are synonymous with high-performance anticorrosive coatings due to the excellent adhesion to metals and high resistance to heat, water, and chemicals they offer. The high chemical resistant nature of a cured film epoxy is attributed to the presence of extremely stable carbon-carbon and ether bonds in the backbone of the epoxy molecule (2). Epoxy coatings are typically used as primers and intermediate coats because they have one significant shortcoming – they suffer from yellowing and chalking upon exposure to ultraviolet radiation. They are very frequently overcoated with a polyurethane topcoat, which protects the epoxy from any degradation, creating an extremely tough anticorrosive system suitable for offshore applications. Although traditionally solvent-based, modern epoxies are available as a water-based system but are known to have inferior anticorrosive properties. This is however a hot topic for coatings manufacturers and recent advances will likely remedy this.
The final category of reaction curing binders is zinc silicates. These are inorganic zinc coatings which have an intrinsic ability to provide cathodic protection. The curing of zinc silicate requires atmospheric moisture and is initiated by a reaction between polysilicon acid and metallic zinc (6), in which the major part of the metallic zinc, which has not reacted, is surrounded by an insoluble matrix of zinc silicate. Due to the moisture-curing reaction requiring atmospheric water to take place, a certain minimum humidity level is required for application (2). Zinc silicate coatings are unique because they offer excellent protection to steel substrates despite defects in the coating surface, such as scratches, pinholes or voids. Further, zinc silicate coatings are typified by having excellent mechanical properties, with good resistance to impacts, scratching and abrasion. They are also good for high-heat applications, and (when appropriately formulated) are suitable for immersion in saltwater. However, they do require care when applying, typically done by specialists.
Selecting a coating system
The selection of protective coatings for the marine and offshore environments strongly depends on the substrate, exposure zone, consideration of specific environmental factors (UV-radiation, flexibility, chemical resistance, etc.) and intended lifespan and maintenance regime to ensure adequate protection of the structure. An example of typical coating systems employed in offshore applications, highlighting the desirable properties for each, is compiled in Table 3.

Table 3: Typical coating systems employed for offshore applications (5)
Consideration also needs to be given to the repair of these coating systems. Being an offshore environment, it can be very difficult, expensive, and very likely to be contaminated with chloride ions during the repairing procedure. It is, therefore, necessary to consider the impact of contamination on these coating systems (11).
Conclusion
Performance coatings represent an enormous field of technologies, chemistries, standards and applications. The variety of existing technologies is vast, and this article has only touched on the most relevant to offshore applications. Looking ahead, it would be prudent to consider the impact of meeting environmental regulations and reducing production costs, both of which are going to become increasingly relevant when designing, manufacturing and applying modern anticorrosive coatings. The current challenge facing formulators and manufacturers is to find environmentally friendly and cost-effective solutions that don’t sacrifice any of the performance of known and trusted traditional formulations. This is further complicated by the incomplete scientific understanding of some corrosion mechanisms, as well as the interaction of certain coating components with their environment. The research aimed at gaining a thorough understanding of degradation mechanisms of substrates and coatings alike, and correlating mathematical models and accelerated exposure testing with natural exposure testing and real-world learnings will provide a useful tool in the development of new environmentally friendly, high-performance products.
About EPCM
References
- Koch, Gerhardus, Brongers, Michiel and Thompson, Neil. Corrosion costs and preventative strategies in the United States. Houston : NACE International, 2002.
- Anticorrosive coatings: a review. Sørensen, P.A., et al. 2, s.l. : Journal of Coatings Technology and Research, 2009, Vol. 6, pp. 135-176.
- Khalifeh, Alireza. Stress Corrosion Cracking Damages. [book auth.] Zheng-Ming Huang and Sayed Hemeda. Failure Analysis. s.l. : IntechOpen, 2019.
- International Organisation for Standardisation. Paints and varnishes — Corrosion protection of steel structures by protective paint systems, Part 2: Classification of Environments. 1998.
- Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. López-Ortega, Ainara , Bayón, Raquel and Luís Arana, José . 1325, s.l. : Materials, 2019, Vol. 12.
- Sander, Jörg, et al. Anticorrosive Coatings. Hanover : Vincentz Network, 2010.
- Winkelaar, Adrie. Coatings Basics. Hanover : Vincentz Network, 2009.
- Gysau, Detlef. Fillers for Paints. Hanover : Vincentz Network, 2011.
- Yebra, DM and Weinell, CE. Key issues in the formulation of marine antifouling paints. [book auth.] Claire Hellio and Diego Yebra. Advances in Marine Antifouling Coatings and Technologies. s.l. : Woodhead Publishing, 2009.
- U.S. Department of the Interior. Review of Corrosion Inhibiting Mechanisms in Coatings. s.l. : U.S. Department of the Interior, 2017.
- Corrosion Protection Systems and Fatigue Corrosion in Offshore Wind Structures: Current Status and Future Perspectives. Price, Seth and Figueira, Rita. 25, s.l. : Coatings, 2017, Vol. 7.

 To all knowledge
To all knowledge