Corrosion Protection of Offshore Facilities: Corrosion is a naturally occurring phenomenon commonly defined as the deterioration of a substance (usually a metal) or its properties because of a reaction with its environment. Like other natural hazards, corrosion can cause dangerous and expensive damage to everything from offshore structures and pipelines to ship hulls and superstructures, inner-hull tanks in fuel tankers, underwater pipes, etc. According to the current NACE ‘Cost of Corrosion’ study, the direct cost of metallic corrosion is $276 billion annually in the US (1). Breaking down this number, however, displays the magnitude of coatings’ role in preventing corrosion. Various technologies are designed to minimise corrosion, from coatings to cathodic protection, advanced materials design, chemical inhibition, etc. The Cost of Corrosion study found that the cost of these technologies totals over $121 billion per year. Of that, however, 108 billion dollars is spent in the coatings service sector. This means there is constant pressure on the coatings industry to stay at the forefront of corrosion prevention technologies, providing cost-effective solutions in the harshest conditions. Advances in coating technology can offer significant cost savings and performance advantages if developed, specified, applied and maintained correctly.
Furthermore, the protective coating (PC) industry is entering a new era, with legislation being introduced internationally to reduce the emission of volatile organic compounds (VOCs) from coatings. To meet the new VOC standards, traditionally effective and trusted corrosion-inhibiting coatings can no longer be used, and high-performance replacements need to be found and necessitate significant changes in the raw materials and formulation of anticorrosive coatings which have traditionally contained a relatively large amount of organic solvents. To comply with new legislation, the industry is moving towards using water-based coatings, powder coatings and coating systems with high solid contents (2). However, it remains a challenge to replace traditional solvent-based coatings, particularly in harsh conditions completely. Critical to the successful formation of coatings for these applications in marine and offshore sectors is a sound understanding of the environment in which it will be applied, the interaction of the coating with the environment, the interaction of the components of the coating with one another, and the potential failure mechanisms for a coating system in a harsh environment.
To better understand the impact of each of these areas, this article includes a general classification of the most common types of corrosion and their failure mechanisms, a description of the expected environments an anticorrosive coating is likely to encounter, an overview of the different families of anticorrosive coatings available including their primary components and working mechanisms, and new coating systems appearing on the market.
The Basics Corrosion Protection of Offshore Facilities – What Is Corrosion, and Why Does It Happen?
It’s common for metallic structural components to experience some form of corrosion regardless of the material used. Corrosion-resistant coatings are designed to increase the lifespan of a part while reducing ongoing maintenance and replacement costs. However, in selecting the most appropriate coating for an application, it is necessary to identify the type of corrosion a structure, part or piece of equipment is prone to. Based on how a part is designed to be used and the conditions it’s exposed to, the kind of corrosion and the required prevention mechanism differ greatly.
While there are many different types of corrosion, there are eight general types of corrosion defined by Fontana and Greene (1967), all of which are relevant to offshore infrastructure. They can be classified by the form in which each manifests itself and can typically be identified by the naked eye. Valuable information for the solution of a corrosion problem can typically be obtained through careful observation of test specimens, failed equipment, or systems currently in the field. Note also that sometimes observed corrosion is not attributable to a single cause but can sometimes result from several interrelated causes.
The first and most common type of corrosion is a uniform attack, characterized by a chemical or electrochemical reaction which proceeds uniformly over the entire exposed surface. Failure usually results from the thinning of the metal over time. The uniform attack represents the greatest metal destruction on a tonnage basis but is also one of the more easily addressed forms of corrosion. Estimating the corrosion rate accurately through simple comparative testing is also possible.
Another commonly encountered form of corrosion is galvanic corrosion. Any time two dissimilar metals are coupled in a solution, a potential difference exists, making one of the metals a cathode and the other an anode. This drives the flow of electrons between the two metals. The net result is that the anode’s corrosion is accelerated while the cathode corrodes at a reduced rate (when compared with its normal corrosion rate). This phenomenon forms the basis of cathodic protection systems.
Crevice corrosion is a slightly less obvious form of corrosion, synonymous with intense localized corrosion occurring frequently within cracks, crevices, and other shielded areas on metal surfaces. This attack is usually associated with small volumes of the stagnant solution caused by holes, gasket surfaces, lap joints, surface deposits, and crevices under bolt and rivet heads.
Pitting is another form of localized attack that typically results in holes or cavities in the metal, small in size. Pits can be isolated or grouped close together, making a rough surface appear. Pitting is one of the most destructive and insidious forms of corrosion. It can cause material failure with only a small per cent weight loss of the entire structure due to perforation. Due to small and easily obscured pits, they are also extremely difficult to detect or measure the extent of pitting quantitatively. Pits are also difficult to analyse under test conditions, making the rate of corrosion particularly difficult to forecast, further exacerbating the danger pits present.
A far less common form of corrosion is intergranular corrosion, the localized attack along the grain boundaries or immediately adjacent to grain boundaries without any significant effect on the grains. Since it only takes place at the grain boundaries of metals, it is largely inconsequential in most applications. However, grain interfaces are very reactive under certain conditions and intergranular corrosion results.
A similar type of corrosion is selective leaching, also known as dealloying, which removes one component from an alloy by corrosion processes. A common example is the selective removal of zinc in brass alloys, which occurs similarly in other alloy systems from which aluminium, iron, cobalt, chromium, or other elements are removed.
Erosion corrosion accelerates the corrosion rate due to a corrosive fluid’s flow over a metal surface. The relative motion of the metal surface and corrosive fluid causes turbulence, which can result in rapidly increasing erosion rates. Typically characterized by the appearance of surface grooves, gullies, waves, rounded holes, and valleys in a directional pattern, erosion-corrosion is sometimes caused by poorly finished surfaces or poor workmanship. In many cases, material failure due to erosion-corrosion is unexpected. It happens relatively quickly, usually because the predicted corrosion was evaluated under static conditions and flow-driven erosion effects were not considered.
The final form of corrosion is known as stress corrosion cracking (SCC), defined as the growth of cracks due to the simultaneous application of stress and surface exposure to a specific corrosive medium, leading to unexpected catastrophic failure of ductile metals. Cold forming, welding, heat treatment, machining and grinding can all introduce residual stresses, the magnitude and importance of which are often underestimated. The SCC sites on the metal surfaces may not be visible by visual inspection, while metal parts are filled with microscopic cracks. These invisible cracks progress rapidly, leading the components and structures to catastrophic failures (3).
While understanding the different forms of corrosion is important in preventing them, knowing the environment in which the infrastructure will reside is equally important.
Corrosion Protection of Offshore Facilities: The Corrosive Environment
High-performance anticorrosive coatings are applied to areas within various environments, introducing several unique conditions the coating needs to be resistant against. Some of these include constant or partial immersion in water or soil, atmospheric pollution in industrial settings, and ultraviolet radiation in outdoor cases. The variety of environmental factors and the exposure time to these factors heavily impact the coating requirements and, therefore, formulation, making knowledge of the environment paramount to the successful coating selection.
To better understand the corrosive conditions a coating is likely to encounter, ISO 12944 ‘‘Coatings and varnishes—Corrosion protection of steel structures by protective coating systems’’ presents a classification system that divides environments into three broad types of exposure: immersion, atmospheric and splash zone.
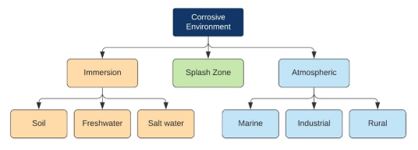
Figure 1: Classification of corrosive environments (2)
Immersion applies to any structures immersed in water or soil. In these cases, the severity of the environment is extremely specific because of the multitude of factors that can play a role in overall corrosivity. Salinity, pH, the presence of dissolved gases, and temperature, amongst others, are all known to contribute to creating a corrosive environment. Due to the wide variety of conditions that can be encountered, ISO 12944 classified them into three categories, as per Table 1.

Table 1: Immersion Categories for Soil and Water (4)
The soil type, pH, humidity, oxygen content, and presence of bacteria and salts mainly determine the corrosivity of soil on buried structures and coatings. The factors for the two aqueous environments also differ significantly from one another. The corrosivity of a freshwater environment is largely defined by the type and concentration of dissolved salts and oxygen (5), along with the expected flow. Higher flow regions introduce a mechanical aspect, with coatings needing to resist erosion and impact from suspended solids. In comparison, salt water has a far higher concentration of salts, particularly chloride, which are extremely aggressive towards coatings and metals. Also requiring consideration for both salt and freshwater is the potential for biofouling (2) and the effect this can have on the performance of the coating.
Atmospheric environments can vary greatly and have alternating conditions, controlled by heat, light, ultraviolet radiation, and relative humidity, along with more constant factors like chemical concentrations, pollution levels, proximity to moisture and distance to the sea. The relative combination of these factors creates a wide range in the environment’s corrosivity, defined by six categories ranging from C1 to C5-I/M based on expected mass and thickness loss for steel and zinc (Table 2). These classifications are somewhat subjective, and a location could fall inside a number of the categories depending on specific conditions. However, they do provide a simple frame of reference when areas are being evaluated for coating application in mind.

Table 2: ISO 12944-2 Classification of Corrosive Environments (4)
The need to create these categories is driven by the different environments having different contributing factors to corrosion and, subsequently, different corrosion mechanisms. For coatings to be suitable for a specific area, they need to be resistant to the dominant corrosion mechanism in the area. For example, rural environments typically have low levels of corrosivity when compared with heavy industrial or marine/offshore environments. Industrial environments are typically classified by high levels of airborne particles, including but not limited to soot, sand and sulphate salts. Also of concern are higher than average levels of gases such as sulphur dioxide, which, when combined with rain, can create an extremely acidic environment. Marine and offshore environments have very high chloride ion content, which is aggressive towards metals and may cause pitting corrosion (2). Therefore, these three environments have very different requirements when evaluating an anticorrosive coating system and must be considered separately.
The final environment, particularly relevant to offshore structures, is the ‘splash zone’, characterised by intermittent wetting and drying due to waves, wind or tides. Corrosion rates in this zone are typically the highest of all the zones, driven by several factors. Firstly, the constant splashing aerates the water, increasing the availability of dissolved oxygen for the electrochemical reactions. Secondly, the cyclic wetting and drying increase the concentration of chloride ions on the surface, again accelerating the corrosion (5). Finally, and of particular concern for coatings, is the exposure to ultraviolet radiation and the abrasive effects of seawater containing sand and other solid debris contributing a mechanical component to the coatings’ deterioration in this environment.
Corrosion Protection of Offshore Facilities: Anticorrosive Coatings
Protective coatings represent one of the simplest ways to reduce corrosion, largely by limiting the exposure of the metal to a corrosive environment. By adhering to the substrate, the coating prevents the corrosive elements from coming into direct contact with the metal. Protective coatings can be sprayed, rolled or brushed on, plated on, or applied using hand tools. Besides corrosion resistance, protective coatings can also be fundamental in allowing a substrate to withstand issues such as wear and exposure to water. They can also improve the asset’s aesthetic appearance.
Anticorrosive coatings vary depending on the type of metal involved, the application area, the predicted exposure the metal will experience during its lifespan, and the corrosion prevention needed. For example, the prevention of galvanic corrosion in iron and steel alloys is typically achieved through the use of zinc and aluminium coatings. Large components, such as bridges and energy windmills, are often treated with zinc and aluminium corrosion-resistant coatings because they have been proven to provide reliable long-term corrosion prevention.
In addition to zinc and aluminium coatings, nickel-chromium and cobalt-chromium are often used as corrosive coatings because of their low porosity. They are extremely moisture resistant and, therefore, help inhibit the development of rust and the eventual deterioration of the metal. Oxide ceramics and ceramic metal mixes are examples of coatings that are strongly worn-resistant in addition to being corrosion-resistant.
A typical anticorrosive coating system for marine and offshore environments (C5-M/CX) usually consists of a primer, one or several intermediate coats, and a topcoat (6). The primer has two main functions, the first of which is to ensure good adhesion of the coating system to the substrate. Without excellent adhesion, no amount of anticorrosive properties will have any effect on protecting the metal, and failure of the coating system is almost guaranteed. Secondly, the primer also protects from corrosion. Primers typically contain anticorrosive additives such as metallic zinc or specialist pigments to provide this functionality. The intermediate coats are generally applied to build up the thickness of the coating system and impede diffusion from the surface to the substrate it is protecting. Increasing the thickness creates a longer diffusion path and provides mechanical strength to the system by distributing stresses.
The intermediate coat must also ensure good adhesion between the primer and the topcoat. Finally, the topcoat is visible and exposed to the external environment. It is designed to provide the surface with the required aesthetics (colour and gloss), resistance to weathering, mechanical impacts and abrasion, and excellent resistance to ultraviolet radiation.
Many factors contribute to an anticorrosive coating, and all need to be assessed to ensure the coating system’s successful performance. Figure 2 illustrates some of the most significant factors that must be considered.
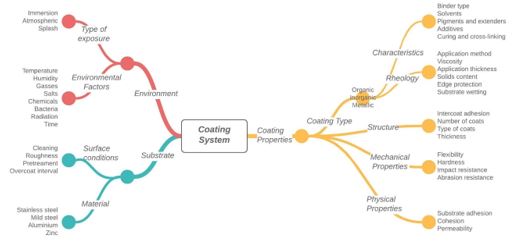
Figure 2: Factors affecting coating system performance (modified from (2))
Typically, anticorrosive systems are classified by how they protect with the substrate, with three major classes of protection existing: barrier protection, inhibitive effect (also known as passivation) and galvanic effect (or sacrificial protection). Barrier protection is simply where the coating system acts as a layer with very low permeability for liquids, gases, and ions, the most important of which is organic coatings. Barrier coatings are typically specified on immersed structures and applied as a conventional multilayer system (primer, undercoat and topcoat). They typically contain inert pigments such as coated titanium dioxide or iron oxide and very low pigment volume concentrations (PVC) driven by higher polymer content, making the permeability to water and gas very low (2). Also requiring consideration is the degree of crosslinking of the polymer, which creates a more macroscopically intact film resistant to diffusion. However, any polymer film will remain penetrable to some extent by gases and electrolytes due to diffusion and migration. Increasing the film thickness is one possible solution to increase the diffusion length (6). It is typically recommended that for the optimal anticorrosive performance of barrier systems, the film thickness is increased through multiple thin coats rather than a single thicker coat. However, this is not always practicable due to time and cost implications.
When examining the working mechanism of barrier coatings, it is important to note that while acting as a barrier to water and oxygen is important, the major contributor to their anticorrosive functionality is their ionic impermeability, which ensures that any moisture at the coating-substrate interface has a very high electrical resistance. Thus, the conductivity of the electrolyte solution is so low that the transfer of corrosion current between the anode and cathode is minimized, preventing corrosion from taking place on the surface of the substrate (2). These coating systems are typically augmented by cathodic protection and, in most offshore structures, use a sacrificial anode, usually in the form of an aluminium alloy, zinc, or magnesium.
Sacrificial coating systems are designed around galvanic protection to minimise substrate corrosion. The coating is formulated using more electrochemically active metals, such as metallic zinc powder, to protect steel structures. This form of protection, however, requires the coating to be in direct contact with the substrate, thus making this type of coating only suitable as a primer. These coatings are sometimes called anodic coatings because the zinc behaves as a sacrificial anode for the steel. The anti-corrosive performance is entirely dependent on the transfer of galvanic current by the zinc. Still, the metal will be galvanically protected if the system remains conductive and sufficient zinc to act as an anode. Due to the requirement for metallic contact between the substrate and the zinc, these coating systems typically have a very high solids content, up to 95 wt% being metallic zinc. Due to this high zinc content, the coatings have very little polymeric binder, making them vulnerable in terms of strength, adhesion, flexibility and impact resistance. They, therefore, need to be applied with care in appropriate locations.
The final coatings category works by passivating the substrate’s surface, resulting in a build-up of insoluble metallic complexes that act as a barrier to corrosive elements. These coatings also rely on direct contact with the substrate and, as such, can only be applied as primers. They are also typically recommended for atmospheric environments and not for immersion in water or soil.
Main Components of an Anticorrosive Coating
Designing an anticorrosive coating requires a sound knowledge of each of the components in the formulation, the function of each of these components, the intended application of the coating, the required level of performance and lifespan, and economic evaluation to target a price point for the finished formulation. Coatings can be formulated from an enormous variety of chemicals or a combination of different chemicals, and there are usually several ways to achieve the same outcome. To better understand the roles of different components in the formulation, they are broken down into five main categories:
- Pigments – Insoluble particles which have two main roles in a coating: to provide colouration and to impart specialist performance, e.g. corrosion resistance, special effects, etc.
- Binders – Binders are the ‘glue’ holding a coating together. They are polymeric film formers that enable bonding between the pigments and between the surface and the pigments (7). Examples include alkyds, acrylics, epoxies, and polyurethanes.
- Extenders – naturally occurring minerals added to coatings to increase solid content without increasing costs, provide brightness and strength, and control wet-state rheology (8). They do not typically contribute to the anti-corrosive performance. Examples include calcium carbonate, talc, and kaolin.
- Additives – covers a wide variety of chemicals typically added in small concentrations to achieve a certain outcome, e.g. surfactants, wetting agents, biocidal agents, antifoaming agents, anti-settling agents, rheology modifiers, UV light absorbers, curing agents, film-forming solvents,
- Solvents – A volatile liquid consisting of one or more components can dissolve binders and other constituents without needing a chemical reaction (7). Most solvents are volatile under normal application conditions, which makes paint processing suitable for the application.
The pigment, binder, and solvent all play significant roles in anticorrosive coatings and will be discussed separately. Additives represent a huge variety of specialist chemicals designed for specific applications, and the full gamut of additives falls outside the scope of this article.
Corrosion Protection of Offshore Facilities: Anticorrosive Pigments for Offshore Applications
Any discussion of pigments in coatings would be remiss if it didn’t include a definition of the pigment volume concentration (PVC), an important value to be considered when formulating. PVC represents the proportion of pigments and extenders in the dry paint film (Equation 1).

The lower the PVC, the higher the paint quality, as the pigments and extenders represent a lower proportion of the dry film volume, with binder solids taking up a higher proportion. The PVC should always be considered alongside the critical PVC (CPVC), which represents the point at which the system has sufficient binder to entirely coat the discrete pigment and extender particles (9). If the PVC is lower than the CPVC, there is sufficient binder to wet and fill the voids between particles, and the final film is uniform. This is important for corrosion protection as the coating is a barrier to water, oxygen and other corrosive species, and thus, anticorrosive paints are typically formulated to be below CPVC. Alternatively, when the PVC is higher than the CPVC, the presence of air in the voids between particles caused by the insufficient binder dramatically changes the properties of a coating (2). CPVC can be determined experimentally or estimated using the oil absorption of the pigments and extenders.
Pigments which play a role in imparting anticorrosive properties to a coating can be classified into three broad categories: barrier, sacrificial and inhibitive. Barrier pigments typically act physically, using a flat, flake-like lamellar structure with a high aspect ratio to lengthen the diffusion path for corrosive species through the dried paint film. This has the additional benefit of providing internal reinforcement to the paint film, increasing mechanical strength and inter-coat adhesion while reducing UV absorption. Of these pigments, the most widespread is micaceous iron oxide (MIO), a crystalline form of iron oxide (α-Fe2O3). Named because it fractures into thin flakes when dispersed, giving a final appearance very similar to that of mica (6). Coatings using barrier pigments are typically formulated to a PVC of between 25 and 40%, well below CPVC, to achieve optimal barrier properties.
Other commonly found barrier pigments include lamellar forms of aluminium, both leafing and non-leafing varieties. Leafing varieties are typically surface-treated during manufacturing to lower surface tension and cause the pigment to ‘float’ to the surface of the coating (2). For this reason, they typically require overcoating to protect the metallic finish from unwanted corrosion processes and pigment abrasion (6). Non-leafing aluminium pigments orientate randomly and homogeneously throughout the coating, forming extremely effective barrier coatings. Glass flakes are also employed as barrier pigments due to their inert properties. Still, their relatively large size (100-400µm) necessitates their application only in thick coatings. Glass flake technology has been a mainstay of the oil and gas (O&G) industry for many years, particularly for highly aggressive splash zone areas. When coupled with other functional pigment systems and advanced polymeric binders, glass flakes can effectively protect tanks, vessels, pipelines, and immersed or atmospheric structural steelwork.
Sacrificial pigments can be any metallic pigment that is more electrochemically active than the substrate. In practice, only metallic zinc particles are used in this application; however, magnesium is increasingly used to protect aluminium. Zinc in the coating protects a ferrous substrate by acting as a sacrificial anode, which corrodes in favour of the ferrous cathode. Particle size is critical to the performance of sacrificial pigments, with smaller particles offering a far greater surface area for reaction. The particle shape and the width of the particle size distribution are also important. Non-uniform shape and a broad distribution offer improved packing compared with spherical particles of a single size. Improved packing reduces porosity and permeability and increases the number of electrical contact points, which improves the ability of the coating to conduct the galvanic current away from the substrate. Coatings containing zinc dust are widely used in organic and inorganic matrices to protect structural steel, offshore and marine coatings and underwater steel constructions (6).
Corrosion-inhibiting pigments (also known as active pigments) are the most complex corrosion protection pigments and function by disrupting the corrosion reaction typically through a competing reaction. Still, the exact mechanism or reaction depends on the specific pigment type. Coatings utilizing inhibitive pigments release soluble species from the pigment into the electrolyte once it has saturated the coating. These species then inhibit corrosion in passivation by facilitating the growth of insoluble protective layers on the substrate surface. Passivation refers to forming thin oxide layers and the metallic component losing its reactivity (10). Some of the most popular inhibitive pigments are either lead-containing or chromates, which are widely used and known to be extremely effective corrosion-inhibiting pigments. However, manufacturers are actively seeking replacements due to the toxicity and known carcinogenic nature of these pigments and the introduction of legislation to control their use very tightly. Of these, inorganic phosphates, molybdates, and silicates are gaining acceptance and are seeing increased application in anticorrosive coating systems.
Corrosion Protection of Offshore Facilities: Solvents
Another major component of any paint is the solvent, which is only intended to be in the film temporarily while it dries. However, solvents play several important roles, including dissolving, dispersing or wetting other components, reducing the viscosity to allow for application, and slowing the drying rate for improved film formation. Coatings are normally classed based on the highest concentration solvent, whether water-based, solvent-based, or solvent-free. As stated earlier, there is a major drive to move towards environmentally friendly formulations and to avoid dependence on solvent-based coatings, particularly in the performance coating sector.
However, this is not without complications. The solubility of the polymeric binders traditionally used in anticorrosive paints is far lower in water than in organic solvents, making dissolution of the polymer problematic. New developments in binders suitable for application in water-based anti-corrosive coatings are styrene-butadiene, vinyl acrylic copolymers, and chemically-cured epoxy binders. Water-based systems also have several inherent drawbacks when compared with their solvent-based counterparts, the first of which is the need to counteract the occurrence of flash rust. Other considerations include their inability to endure freeze/thaw cycles, any restrictions on atmospheric humidity during application and curing, and susceptibility to wet-state biological attack from bacteria and fungi. All of these are particularly significant for offshore applications, where storage and application conditions cannot be easily controlled. Thus, the benefits of using a water-based coating system need to be carefully weighed against the increased potential for challenges during application and long-term durability concerns.
An interesting coating category is ‘solvent-free’ systems, essentially 100% solid powder-based systems. These are applied electrostatically and then baked using thermoset or thermoplastic polymers to provide a durable protective finish. This is an environmentally friendly alternative to solvent-based coatings, as the formulations contain no solvents. However, these coatings require baking after application to form an impervious layer, requiring additional infrastructure for the application. This drastically limits the size of the items that can be powder coated, meaning limited application for offshore infrastructure. A further issue is difficulty with maintenance, as powder coatings can’t be overcoated or touched up.
Traditional solvent-based coatings have used a wide range of organic liquids, such as aromatic and aliphatic hydrocarbons, glycol esters, and alcohols. However, the correct choice of solvent technology for anticorrosive coatings depends on the required properties of the system’s binder, as well as the environment, method of application, curing, pigmentation, and nature of the substrate.
Corrosion Protection of Offshore Facilities: Common Binders for Anticorrosive Coatings
The binder is responsible for several vital characteristics of a coating, including adhesion to the substrate, cohesion within the coating, mechanical strength, flexibility, durability, resistance to abrasion and resistance to permeability. These properties form as the applied coating transitions from wet to dry, as the individual polymer particles crosslink when curing. Film formation occurs in one of three ways: drying (evaporation of the solvent from the film), chemical reaction, or a combination of both (Figure 3).
The first and extremely common family of reaction-cured binders is alkyds. The etymology of the word alkyd is derived from alcohol and a-CID, later changed to alkyd (6). Alkyd-based coatings are characterised by good adhesion, flexibility, resistance, and durability. Alkyd coatings are often employed as primers, typically with an inhibitive pigment in corrosive environments. However, the greatest weakness of alkyd coatings is the potential for saponification, where alkaline materials attack ester linkages in the resin to form an alcohol and the salt of a carboxylic acid. Saponification is particularly problematic when alkyd primers are applied on galvanized surfaces, in zinc-rich primers, or when applied to systems with cathodic protection or sacrificial anodes (2).
The next category, polyurethane coatings, is typified by their excellent resistance to weathering and resistance to UV radiation, making them particularly effective as topcoats for offshore applications. They can also be formulated to be somewhat ‘self-healing’ due to the ability of the hydrogen bonds between the urethane linkages to reform after being broken (2). Polyurethanes are available as two-component solvent-based coatings, which are the reaction product of isocyanates with compounds containing an active hydrogen atom (amine or hydroxyl group). Water-borne polyurethanes are also available and are typically two-component systems based on separate dispersions of polyols and isocyanates in water. Compared to two-component solvent-borne coatings, water-borne urethane coatings are lacking in chemical and corrosion resistance and not frequently used in anti-corrosive applications (2).
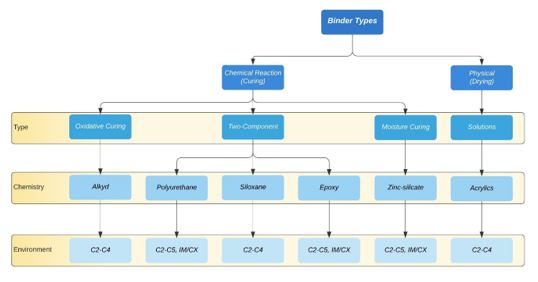
Figure 3: Classification of the binder by curing mechanism and potential application environments (adapted from (2))
Siloxane systems contain polysiloxanes, polymers with a silicon-oxygen (Si–O) backbone and made from monomeric building blocks. Compared with traditional organic binders, polysiloxane coatings exhibit superior gloss and colour retention but have poor mechanical properties (2). A key strength of silicon-based polymers is their inherent temperature-resistant nature. Other strengths include resistance against UV irradiation and oxidation, which have motivated attempts to use the chemistry for property improvement of both organic and inorganic systems, including good resistance to certain acids and solvents, high nonvolatile content and good weather ability (6). Further, because the siloxane binder is already oxidised, it is impervious to further oxidation. Siloxanes can also be reacted with epoxy, acrylic or other organic compounds to form hybrid siloxane coatings, which have found widespread usage for industrial applications because of the improved adhesion and resistance to moisture brought about by the siloxane (2).
Epoxy resins are synonymous with high-performance anticorrosive coatings due to their excellent adhesion to metals and high resistance to heat, water, and chemicals. The highly chemical-resistant nature of a cured film epoxy is attributed to the presence of extremely stable carbon-carbon and ether bonds in the backbone of the epoxy molecule (2). Epoxy coatings are typically used as primers and intermediate coats because they have one significant shortcoming – they suffer from yellowing and chalking upon exposure to ultraviolet radiation. They are frequently overcoated with a polyurethane topcoat, which protects the epoxy from any degradation, creating an extremely tough anticorrosive system suitable for offshore applications. Although traditionally solvent-based, modern epoxies are available as water-based systems, known to have inferior anticorrosive properties. This is, however, a hot topic for coatings manufacturers, and recent advances will likely remedy this.
The final category of reaction-curing binders is zinc silicates. These inorganic zinc coatings have an intrinsic ability to provide cathodic protection. The curing of zinc silicate requires atmospheric moisture. It is initiated by a reaction between polysilicon acid and metallic zinc (6), in which the major part of the metallic zinc, which has not reacted, is surrounded by an insoluble matrix of zinc silicate. Due to the moisture-curing reaction requiring atmospheric water to take place, a certain minimum humidity level is required for application (2). Zinc silicate coatings are unique because they offer excellent protection to steel substrates despite defects in the coating surface, such as scratches, pinholes or voids. Further, zinc silicate coatings are typified by having excellent mechanical properties and good resistance to impacts, scratching, and abrasion. They are also good for high-heat applications and (when appropriately formulated) are suitable for immersion in saltwater. However, they do require care when applying, typically done by specialists.
Selecting a Coating System
The selection of protective coatings for the marine and offshore environments strongly depends on the substrate, exposure zone, consideration of specific environmental factors (UV-radiation, flexibility, chemical resistance, etc.) and intended lifespan and maintenance regime to ensure adequate structure protection. An example of typical coating systems employed in offshore applications, highlighting the desirable properties for each, is compiled in Table 3.

Table 3: Typical coating systems employed for offshore applications (5)
Consideration also needs to be given to the repair of these coating systems. Being an offshore environment, it can be very difficult and expensive, and it is likely to be contaminated with chloride ions during the repair. It is, therefore, necessary to consider the impact of contamination on these coating systems (11).
Conclusion
Performance coatings represent an enormous field of technologies, chemistries, standards and applications. The variety of existing technologies is vast, and this article has only touched on the most relevant to offshore applications. It would be prudent to consider the impact of meeting environmental regulations and reducing production costs, both of which will become increasingly relevant when designing, manufacturing and applying modern anticorrosive coatings. The current challenge facing formulators and manufacturers is to find environmentally friendly and cost-effective solutions that don’t sacrifice the performance of known and trusted traditional formulations. This is further complicated by the incomplete scientific understanding of some corrosion mechanisms and the interaction of certain coating components with their environment. The research aimed to understand the degradation mechanisms of substrates and coatings alike, correlating mathematical models and accelerated exposure testing with natural exposure testing and real-world learnings, which will provide a useful tool in developing new environmentally friendly, high-performance products.
About EPCM
References
- Koch, Gerhardus, Brongers, Michiel and Thompson, Neil. Corrosion costs and preventative strategies in the United States. Houston : NACE International, 2002.
- Anticorrosive coatings: a review. Sørensen, P.A., et al. 2, s.l. : Journal of Coatings Technology and Research, 2009, Vol. 6, pp. 135-176.
- Khalifeh, Alireza. Stress Corrosion Cracking Damages. [book auth.] Zheng-Ming Huang and Sayed Hemeda. Failure Analysis. s.l. : IntechOpen, 2019.
- International Organisation for Standardisation. Paints and varnishes — Corrosion protection of steel structures by protective paint systems, Part 2: Classification of Environments. 1998.
- Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. López-Ortega, Ainara , Bayón, Raquel and Luís Arana, José . 1325, s.l. : Materials, 2019, Vol. 12.
- Sander, Jörg, et al. Anticorrosive Coatings. Hanover : Vincentz Network, 2010.
- Winkelaar, Adrie. Coatings Basics. Hanover : Vincentz Network, 2009.
- Gysau, Detlef. Fillers for Paints. Hanover : Vincentz Network, 2011.
- Yebra, DM and Weinell, CE. Key issues in the formulation of marine antifouling paints. [book auth.] Claire Hellio and Diego Yebra. Advances in Marine Antifouling Coatings and Technologies. s.l. : Woodhead Publishing, 2009.
- U.S. Department of the Interior. Review of Corrosion Inhibiting Mechanisms in Coatings. s.l. : U.S. Department of the Interior, 2017.
- Corrosion Protection Systems and Fatigue Corrosion in Offshore Wind Structures: Current Status and Future Perspectives. Price, Seth and Figueira, Rita. 25, s.l. : Coatings, 2017, Vol. 7.

 To all knowledge
To all knowledge