1 Introduction to Gas Processing
Natural gas treatment is a technical procedure. The purpose of gas treatment is to clean the gas by extracting contaminants, fluids, and hydrocarbons (non-methane). In oil and gas terminology, processed gas is called pipeline-friendly gas.
Gas composition depends upon the type of producing well, depth, and area’s geology. Gas processing units clean the contaminant-rich gas by eliminating pollutants like hydrogen sulfide, carbon dioxide, and water. Pollutants like carbon dioxide have commercial value; such contaminants are treated further.
2 Gas Processing: Contaminants in Natural Gas
Methane (CH4) is the main constituent of the naturally produced gas. CH4 is the lightweight and shortest molecule present in the naturally produced gas. The gas produced naturally contains different amounts of contaminants, which are:
- Heavy Gaseous Hydrocarbons: normal butane, propane, ethane, isobutane, and pentanes.
- It contains acid gases like CO2, hydrogen sulfide, and mercaptans.
- Other Gases: Helium and nitrogen.
- Naturally produced gas also contains vapours and free water. It may also contain mercury and chlorides.
- It can also contain naturally occurring radioactive materials like radon and traces of radium.
When the contaminants like H2S, CO2, and N2 exist in the naturally produced gas, it is called acid gas or sour gas.
When vapours and water exist in the gas, it is known as wet gas.
3 Objectives of Natural Gas Treatment
The following are the objectives that are achieved by the processing of the naturally produced gas:
- Remove the content of H2S from the naturally produced gas to make it sweet.
- Remove carbon dioxide and meet customer specifications.
- Eliminate the water content until it does not surpass the limit set by the customer.
4 Reasons for Achieving the Objective of Gas Processing:
The following are the reasons to achieve the objectives of gas processing:
- To produce transportable and pipeline-quality gas.
- To achieve sale-gas conditions set by the customer.
- To enhance the liquid recovery.
5 Types of Gas Processing
Raw naturally produced gas is treated to eradicate all contaminants. Two types of gas processing systems are generally used in the E&P industry to remove impurities.
5.1 Glycol Gas Dehydration
As the name dehydration indicates, this process uses glycol adsorption to eliminate the water vapours and water from the produced gas.
5.2 Amine Gas Sweetening
The process of amine sweetening uses amine to eliminate hydrogen sulfide from naturally produced gas and turns acid gas into sweet and pipeline-friendly gas.
6 Glycol Dehydration
The procedure for producing gas dehydration is achieved in two steps. Firstly, gas is dehydrated, and vapours or free water are eliminated via a chemical reaction in the dehydration package’s absorber. The second step is the treatment of water-rich glycol to make it lean glycol for use in the next cycle. Before we begin, let’s talk about the major components of the glycol dehydration unit.
6.1 Components of Glycol Gas Dehydration Unit
a) Inlet Separator
The inlet separator receives the natural gas and removes the free water that comes with the natural gas. The separator can be two-phase or three-phase, depending upon the produced fluid. A gas separator has a mist extractor and wire mesh that separates free water and other particulates from the natural gas.
b) The Contact Tower
The contact tower is the primary workplace of the entire gas dehydration process. In the contact tower, glycol and gas come into contact, dehydrating the gas. Different factors are involved in designing the contact tower, like contact tower height and flow rate of the produced gas.
The stream’s flow is countercurrent in the contact tower. This means the feed gas enters through the bottom and moves upward, while TEG enters from the top and travels downward in the contact tower. When glycol moves downward and gas moves upwards, they come in contact, and glycol absorbs the vapours and water in the feed gas, dehydrating it.
c) The Flash Tank
The second step of the glycol dehydration process involves a flash tank. In the tank, lean glycol is flashed at low pressure, allowing the volatile components present in the glycol to vaporize.
d) Heat Exchanger (Lean – Rich)
The principal objective of the heat exchanger is to conserve energy. In the heat exchange, glycol from regeneration is chilled by the glycol exiting the contact tower. The glycol entering the contact tower should be cool for a chemical reaction to occur properly. The water-rich glycol should be hot to properly strip water vapours from the glycol.
e) The Dehydration Unit Regenerator
The regenerator uses fractionation to separate water from glycol. This process is achieved in the reconcentration vessel’s still column. Due to the alteration in temperature, the glycol condenses, and the liquid water vaporizes.
The size of the regenerator is determined by various factors, including the glycol circulation rate, reboiler temperature, and the volume of water vapours present in the feed gas.
f) Glycol Pumps
Glycol circulation pumps are essential parts of the dehydration unit. They establish glycol circulation, increase the pressure of glycol, and deliver it to the contact tower so that the dehydration process can begin. Typically, two to three glycol circulation pumps are installed on a dehydration unit.
6.2 Gas Dehydration Process
The gas must be dehydrated if the naturally produced gas contains a large volume of water. This is because excess water causes problems like corrosion, gas hydrates, and freezing. Gas must be dehydrated to meet pipeline standards. The detailed process of gas dehydration is explained below:

Figure 1: Glycol Gas Dehydration Unit Flow Chart
The wet gas, rich with water, first enters the two-phase or three-phase inlet separator to separate the water from the gas. If the natural gas contains free water, a three-phase inlet separator must be used to knock out the free H2O. Although a mist remover is installed in the separator, the feed gas still contains a small volume of water vapours.
The wet gas flows into a contactor vessel from the bottom and moves up. The glycol flows into the contact vessel from the top and moves down. The chemical reaction occurs, and the lean glycol becomes water-rich glycol by absorbing the water and vapours present in the wet feed gas, making it the dehydrated gas. Before the dehydrated gas goes through a metering system into the downstream pipeline, the dry gas enters the glycol cooler to lower the temperature of hot glycol before flowing into the contact tower.
The rich glycol exiting from the contact tower flows into a reflux condenser. After the reflux condenser, the water-rich glycol flows into the flash tank, vaporising turbulent and unstable components.
After flowing from the flash tank, the water-rich glycol enters glycol filters and the heat exchanger. Water-rich glycol exchanges heat with the high-temperature lean glycol. It then enters the TEG regenerator with the still column and reboiler, where water is eliminated from the rich glycol via distillation. Moreover, the glycol’s absorption ability is increased to fulfil the TEG requirement. Once the rich glycol becomes the lean glycol, the glycol circulation pumps pump the glycol into the contact tower for the next cycle.
7 Amine Sweetening Process
The amine-sweetening process of the naturally produced gas is accomplished in two steps. Firstly, the raw natural gas is purified, and hydrogen sulfide is removed. Secondly, the rich amine is treated to remove the hydrogen sulfide. Before discussing the procedure of amine sweetening, let’s discuss the surface facilities of the amine sweetening plant.
7.1 Surface Facilities of Amine Sweetening Plant
a) Separator
An inlet separator eliminates contaminants like water, mist, and hydrocarbon condensate and prevents them from entering the amine system. Depending on the fluid produced by the well, two-phase or three-phase separators are used.
b) Absorber
The absorber is the primary place where hydrogen sulfide removal takes place. The sour gas and lean amine are encountered in the absorber. The gas enters from the bottom and flows to the top through the trays. The lean amine enters from the absorber’s top and leaves from the absorber’s bottom. The encounter of acid gas and amine turns acid into sweet gas, and amine becomes rich in acid gas.
c) Treated Gas Knock Out Drum
The knock-out drum (KOD) is equipped with a demister pad, and the dry gas from the absorber enters the KOD. The primary objective of the gas knockout drum is to eliminate the entrained amine coming with the dry gas.
d) Amine Flash Drum
An amine flash drum enables the removal of a portion of acid gas. It is a three-phase horizontal vessel operated at minimum pressure.
e) Heat Exchanger
A heat exchanger conserves energy and reduces the total energy requirements for the amine sweetening process. Low vaporization should occur in a heat exchanger to prevent corrosion/erosion caused by the mixed flow.
f) The Reboiler
The amine solution rich with acid gas leaves the regenerator and flows into the reboiler. The reboiler produces heat energy, which helps separate acid gas from the rich amine and turn it into the lean amine.
7.2 Amine Sweetening Unit Explained
If the naturally produced gas has a large volume of hydrogen sulfide, that gas is called sour and must be treated before it flows into the pipeline. A large volume of H2S in the naturally produced gas is dangerous for equipment and will cause the pipeline to corrode. Hydrogen sulfide must be removed to prevent this damage. The amine sweetening of naturally produced gas accomplishes the elimination of hydrogen sulfide. The detailed working mechanism of the amine plant is explained below:
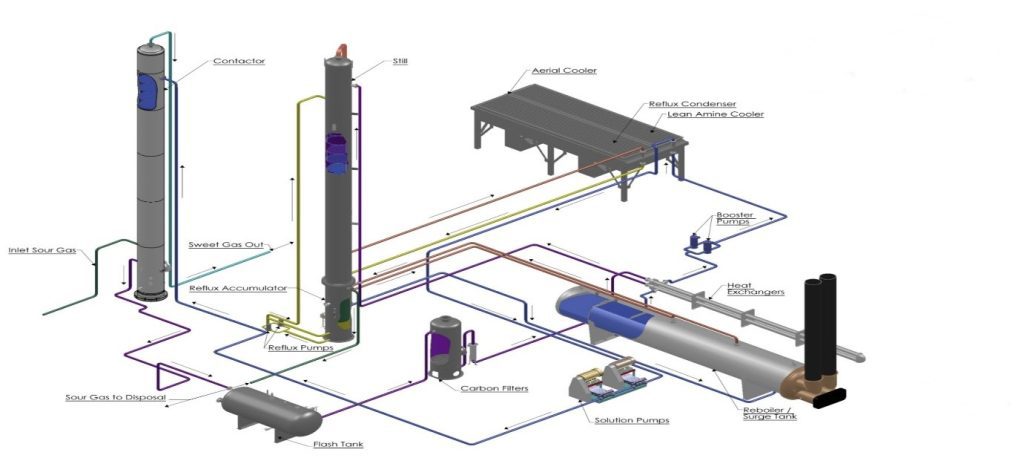
Figure 2: Amine Gas Sweetening Unit Diagram
The raw natural sour gas from the well enters the inlet separator, where contaminants like mist, water, and condensate are removed. The acid gas free from contaminants flows into the absorber from the bottom of the contact vessel and flows upward, while the lean amine solution enters from the top of the absorber and flows downward.
When lean amine and acid gas come in contact, an exothermic chemical reaction occurs. In this reaction, the amine absorbs the acidic contaminants of feed gas and turns sour gas into sweet gas, while the lean amine becomes rich amine after absorbing the acid gas. The treated gas flows out from the upper part of the absorber, while the amine with acid gas flows from the bottom of the absorber. The processed feed gas from the contractor enters the processed gas KOD, where any amine traces in the treated gas are removed and routed towards downstream facilities.
The rich amine solution flows from the absorber bottom into the amine flash drum. In the amine flash drum, the portion of acidic gas is eradicated. The rich amine solution flows out of an amine flash drum and into the heat exchanger. The heat exchanger conserves energy and reduces the energy needed for the sweetening procedure. The temperature of the acid gas-rich amine is enhanced by the lean amine approaching the regenerator.
The amine rich with acid gas then flows into the regenerator, which has a condenser and a reboiler. A reboiler is a direct-fired heater where the temperature of amine rich with acid gas is increased to eliminate all acid gases from the amine solution. The amine is then routed towards the heat exchanger. The regenerator’s product is a combination of acid gas and water vapours, and the condenser installed in the ASU handles this stream. In the condenser, the water vapours condense to produce the reflux.
The reflux drum in the amine plant collects water vapours and acidic gases from the condenser. The acid gas that leaves the reflux drum flows towards the disposal system designed for acid gas. The reflux handles the water from the reflux pump, which pumps it to the regenerator top.
The lean amine that flows out from the heat exchanger still has an increased temperature. Finally, it flows through the lean amine cooler to decrease its temperature. The lean solution cooler uses air to lower the temperature of the lean amine. The lean amine is then sent to the contact tower via circulation pumps for the next cycle.
Mechanical and activated carbon filters are also available on the amine unit. The mechanical filter is placed on the lean amine stream. It filters the solution from solid impurities like sand, iron oxide, iron sulfide, and pipeline dust. The activated carbon filter is installed on the downstream. It filters out water-soluble compounds, unknown foam contributors, and hydrocarbons.
8 Hydrocarbon Dew Point Control Unit
Regulating the hydrocarbon dew point is another important parameter of gas processing to meet pipeline specifications. The temperature at which vapours begin to condense from the gaseous phase is known as the dew point. The hydrocarbon dew point control unit, or HDPCU, regulates the dew point. The HDPCU is installed in cold areas to prevent the condensation of hydrocarbons.

Figure 3: Hydrocarbon Dew Point Control Unit
HDPCUs have the sole purpose of restricting the creation of hydrates in the natural gas stream or pipeline. They perform an equivalent of dehydration, and they are accomplished by injecting the hydrate inhibitor straight into the gas pipeline. Moreover, hydrocarbon dew point control units are developed to use refrigeration or JT (Joule Thomson Effect) to provide the level of cooling as needed.
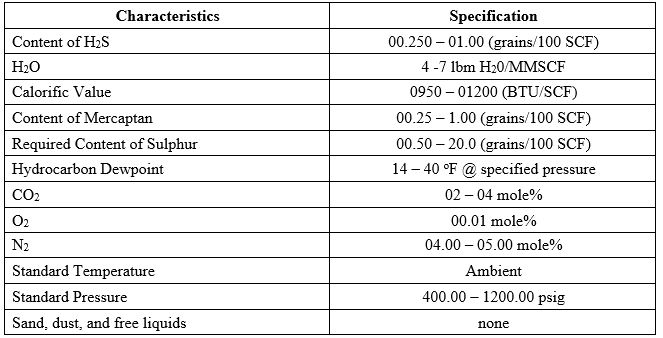
9 Pipeline Gas Specifications
Naturally produced gas must be processed to fulfil the standards of a gas transportation pipeline. The specifications of the gas pipeline are shown in the table below. These pipeline gas specifications ensure that gas is of high quality and that customers get safe and clean gas. Moreover, it should meet the heating values defined in Wobbe’s index to warrant the optimal operation of gas turbines for electricity generation.
10 Gas Processing: Conclusion
Gas processing is essential if there is a large volume of contaminants present in the naturally produced gas. Pollutants like water, H2S, CO2, and N2 can damage the pipeline and equipment. They can cause corrosion and create problems like freezing and hydrates. The gas must be treated to make it pipeline-friendly and transportable. Moreover, the treated gas can avoid economic loss and prevent equipment damage.
More on EPCMs knowledge on Natural Gas.

 To all knowledge
To all knowledge