1 Introduction to Fluidised Bed Reactor Design for Pyrolysis
Pyrolysis is the process where thermal conversion of organic matter occurs using a catalyst in the absence of oxygen, typically to produce liquid fuel. The products of biomass pyrolysis include bio-oil, biochar and Non-Condensable Gases (NCG) including various compositions of hydrogen, carbon monoxide and carbon dioxide depending on the biomass selected (1). The condensed gases collected during pyrolysis result in the final desired product, bio-oil. Pyrolysis has been drawing increasing attention due to its high efficiency and reduced impact on the environment in contrast to the crude oil sector as it creates an opportunity to recover energy from waste materials. Various raw materials may be pyrolysed, for example, used tyres are pyrolysed in some regions of the world. In this article, the pyrolysis of agricultural residues will be analysed.
2 Background
The bio-oil collected during pyrolysis produces approximately 40 MJ kg-1, this is similar to the amount of energy contained in other commercially produced fuels such as crude oil, diesel and petrol which contain 45.5 MJ kg-1, 45.8 MJ kg-1 and 46.6 MJ kg-1 respectively (2).
During pyrolysis woody biomass is converted according to the reactions illustrated below:
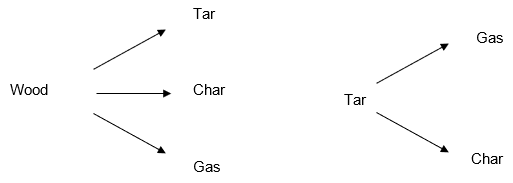
Biochar produced during pyrolysis, as a by-product, enhances crop yield and acts as a soil enhancer providing numerous nutrients to the soil and thus improving crop yield. Excess biochar generated can be sold to the agricultural sector to recover costs. As a result, it is recommended that biochar generated be sold as a soil amendment (3).
For the maximum production of char and gases, the thermal degradation process and conditions should be optimised for maximum bio-oil production for fast pyrolysis (4). For optimum reaction conditions, the following is recommended (5):
- moderate temperatures (optimal temperature taken as 500 °C)
- rapid heating of biomass particles
- short residence time of the pyrolysis vapours
- fast quenching of pyrolysis vapours to condense the bio-oil
Fast pyrolysis typically results in a product distribution of 75 wt.% bio-oil, 12 wt.% char and 13 wt.% gases (5). The bio-oil generated has the following properties: low pH, low heating value, poor, volatility, high viscosity and high oxygen content. The quality of the bio-oil can be enhanced by using a catalyst during the pyrolysis (6).
Char is a solid by-product consisting of carbon, oxygen, hydrogen and Nitrogen. The char yield range is dependent on the pyrolysis temperature and the yields can vary from 10 – 20 wt.%. Coke is formed on the surface of the catalyst during fast pyrolysis. This catalytic coke can result in the deactivation of the catalyst and, as a result, should be removed. This is done by burning it away (1).
2.1 Catalyst selection
Numerous investigations have concluded that catalytic fast pyrolysis optimises bio-oil yield and quality by enhancing the NCG emitted. The amount of char produced is also decreased, which thereby minimises the instability or ageing of the bio-oil (7). Due to the endothermic nature of this pyrolysis reaction, adding a catalyst will decrease the overall process costs and energy consumption, as it lowers the temperature of the reaction. A variety of catalyst choices exist, and the selection is dependent on process feedstock and the pyrolysis process system selected. An LDH catalyst is recommended. The catalyst eliminates the need for bio-oil upgrading and simplifies the production procedure (8).
2.2 Biomass Choice
Eucalyptus was selected as the type of biomass modelled due to its rapid growth rate and abundant supply in South Africa. Also, it contains a smaller percentage of ash and Nitrogen when compared to other types of biomass. (9).
2.3 Heat transfer
The rate of heating directly influences the reaction pathway and substances produced. Rapid heating results in smaller amounts of char. Moreover, the oil yield is affected by the heating rate, which decreases at lower heating rates (10).
The main methods that heat transfer occurs during flash pyrolysis are gas-solid heat transfer using convection and solid-solid heat transfer utilising of conduction. A fluidised bed is beneficial in that 90% of the heat transfer is from conduction and the remainder from convection. Also, due to fluidisation, attrition occurs whereby there is friction between the biomass and the hot catalyst. This erodes the surface of the biomass, thereby exposing fresh biomass for a reaction as well as eroding the carbon layer around the catalyst which can hinder its activity. This can slightly reduce particle size of the biomass. However, the con is that micro carbon is formed, which can prove difficult to remove from the vapour phase and may make up a component of the bio-oil. However, the amount of micro carbon formed is minimal in fluidised beds when compared to other types of pyrolysis reactors (4) The hot sand flows from the combustor to the pyrolyser as illustrated below:
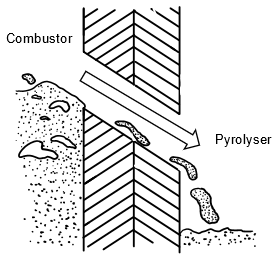
2.4 Residence Times
Vapour residence times of less than 2 s are recommended as longer residence times result in secondary cracking of the primary products leading to reduced yield and negatively influencing the quality of the bio-oil (4).
2.5 Particle Size
A wood particle size of 4 mm was used as recommended by Liao & Thomas (11).
3 Mass and Energy Balance

A mass and energy balance is vital for the sizing of the reactors and needs to be conducted over the enter pyrolysis process as a whole. The moisture content of the biomass should also be known for this mass and energy balance. The chemical formula for the biomass can be obtained in a study conducted by Adebayo et al. (3) For a full layout of the entire pyrolysis production process, refer to the diagram below. This article will illustrate the calculations necessary to size the combustor and pyrolyser, outlined in red.
The main goal for conducting an energy balance in the pyrolyser is to achieve a temperature of 500 °C to enable fast pyrolysis to produce a high-quality bio-oil. The energy required for the pyrolyser is obtained by heating the catalyst, modelled as sand using the software AspenPlus for the simulation, in the combustor which should operate at 900 °C. The catalyst is then fluidised and overflows via the overflow pipe into the pyrolyser for heat exchange with the woody biomass. An energy balance should be first conducted over the pyrolyser to determine the catalyst flow rate required to provide sufficient energy. The diagram below illustrates the main streams for this energy balance.
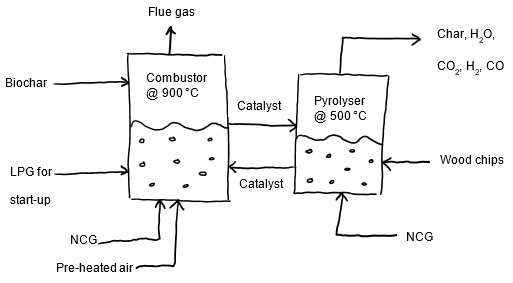
The catalyst (Qcatalyst) needs to provide enough energy to heat the biomass (Qbiomass) and water contained in the wood (Qwater,1) exiting the dryer at a specified temperature and heat it to 500 °C, evaporate the water at 100 °C (Qevap), heat the water from boiling point to 500 °C (Qwater,2) as well as supply energy for pyrolysis (Qpyrolysis) which is an endothermic reaction. Lastly, adequate energy is needed to compensate for energy losses to the environment (QLoss,pyr). Equation 1 illustrates how the above energy requirements are determined.
![]() (1)
(1)
All Q values listed in equation 1 are found using equation 2,
![]() (2)
(2)
Qevap is found using equation 3 and the latent heat of vaporisation ![]() and mass of water vaporised, m.
and mass of water vaporised, m.
![]() (3)
(3)
The energy needed to heat the catalyst (Qcatalyst) is supplied by the combustor, which burns both NCG and biochar to provide this energy. In the NCG, the hydrogen (QH2 in NCG) and carbon monoxide (QCO in NCG) combust to provide energy and, in the biochar, the carbon (QC in char) and hydrogen (QH in char) also combust to provide energy.
The amount of biochar needs to: provide sufficient energy to heat the sand from 500°C to 900 °C (QNCG), heat up the NCG exiting the heat exchanger from 500 °C to 900 °C, heat the air from 25 °C to 900 °C (Qair) and heat up the carbon dioxide (Qco2) and water vapor (QH2O) formed from the combustion of bio-char at the specified temperature it enters at to 900°C and provides energy to recompensate for the heat loss to the environment, QLoss,comb. The bio-char flow rate is varied until the energy balance is satisfied.
The amount of NCG selected is a fixed variable and based on the volumetric flowrate of gas entering the bed to provide a suitable operating velocity and a sized diameter of the bed as explained in the Fluidised Beds section of this article.
 (4)
(4)
Qcatalyst, QNCG, Qair, Qco2 and QH2O are determined according to equation 2. QLoss,comb is found as shown in the Insulation section. QC in char, QHinchar, QH2 in NCG and QCO in NCG are found by multiplying the molar flowrate by the heat of reactions. Equation 4 shows the energy balance.
4 Fluidised Bed Reactor Design for Pyrolysis: Fluidised Bed Design
The process consists of fluidised beds, namely the Combustor and the Pyrolyser as depicted in the figure below:

Figure 1: Schematic of fluidised beds adapted from Swart (12).
The operating velocity, height, diameter, wall thickness as well as the distributor plate are designed according to the procedure below (13) using the values for the density, and viscosity, to be calculated for the relevant components at the specified temperature and pressure of 101,325 kPa.
The assumption that the char particles immediately react to form flue gases in the combustor and that the wood chips instantly pyrolyse due to fast pyrolysis to form NCG is made. Therefore, the following calculations are done by only considering the solid catalyst particles which are present throughout fluidisation in the circulation fluidised beds. According to the Geldhart chart, the sand was classified as a group B powder. Thus the relevant equations for group B powders were used below.

Figure 2: Geldhart powder categorisation (14)
The Archimedes number, the ratio of viscous forces within the bed compared to outside the bed, is calculated according to equation 5, this is used in equation 6, (Wen and Yu (15) to solve for the Reynolds number at minimum fluidisation (Remf). Using Remf it is possible to solve for the velocity at minimum fluidisation (umf) in equation 7 which is the start of fluidisation.
 (5)
(5)
![]() (6)
(6)
 (7)
(7)
Increasing the velocity beyond this results in the creation of bubbles. If the velocity is increased more significantly, the velocity will surpass the terminal velocity (ut) calculated using equation 8, which is the velocity to transfer particles out of the bed. Further increasing the velocity results in entrainment. The operating velocity, uop, should be greater than umf but smaller than ut.
 (8)
(8)
The porosity at minimum fluidisation ![]() is the bed voidage at the instant fluidisation occurs. It can be solved according to equation 9. The height at minimum fluidisation, Hmf, assumes that the extra height of the particles at fluidisation compared to without fluidisation is the height of the particles in equation 10.
is the bed voidage at the instant fluidisation occurs. It can be solved according to equation 9. The height at minimum fluidisation, Hmf, assumes that the extra height of the particles at fluidisation compared to without fluidisation is the height of the particles in equation 10.
 (9)
(9)
 (10)
(10)
The slugging velocity, ums in equation 11, is the velocity at which the bubbles created to have the same diameter as that of the bed. The operating velocity should be below the slugging velocity.
![]() (11)
(11)
The operating Reynolds number is determined according to
 (12)
(12)
Where the operating velocity is selected to meet the criteria outlined in equation 13. Furthermore, an appropriate range of operating velocities for fluidised beds is in the range 1 ms-1 to 3 ms-1 (16).
![]() (13)
(13)
The pressure drop across the bed ![]() is found by equation 14 where g is 9.81 ms-1 and hp is the height of the particles in the bed.
is found by equation 14 where g is 9.81 ms-1 and hp is the height of the particles in the bed.
![]()
![]()
The pressure drop across the distributor, ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()


Corr is 0.6. It should be ensured that the orifice velocity is greater than 50 m s-1 to prevent particle backflow through the orifice (12).
The number of orifices per bed area, Norr, is found by equation 16. Multiplying the bed area by the number of orifices enables the total number of orifices to be found.
![]()
![]()
The height of the beds is calculated using equation 17 and 18 to determine the total disengagement height to find the height to place the overflow pipe. The figure below illustrates the zones calculated when determining the bed height. The region depicted “Bed” is the packed bed height.


Figure 3: Height regions in a fluidised bed (14)


![]()
![]()
In equation 17, dbv is the diameter of a bubble at the surface. The total heights should be multiplied by a safety factor of 1.2, and the overall height for both beds is selected as the height of the taller bed.
The thickness of the walls is important to ensure that the walls can withstand the pressure created. Equations 19 and 20 allow the calculation of the appropriate wall thickness due to hoop stress and longitudinal stress, respectively. P is the design pressure of 101.325 kPa for both beds, S is the maximum allowable yield stress of stainless steel and E is the joint efficiency which is approximated to be 0.6. The final minimum wall thickness is the sum of these two values.
![]()
![]()


5 Fluidised Bed Reactor Design for Pyrolysis: Insulation
Insulation has demonstrated to be advantageous in the following circumstances:
- Decreasing energy costs
- Improving the safety of employees working in hot environments
- Temperature control of equipment
- Decrease in the utilisation of natural resources
- Decrease in pollution as a result of noise.
Insulation materials should be selected based on the service temperature range of the materials, whether they would react with the raw materials and their combustibility. Furthermore, the thickness choice should be based on the thickness of the material typically available from suppliers (17,18).
Protection of insulation is crucial for the longevity of the materials. As a result, firebrick clay is recommended as the outermost layer of insulation, both to protect the inner insulation as well as to provide an additional layer of insulation (18).


The Qloss used in the energy balance is determined according to equation 25 outlined below. In equation 21, RinsulatorX refers to the outermost layer of insulation. The figure below depicts an example of materials used in combination with equation 21![]()
![]()
Heat transfer by convection and conduction are modelled according to equations 22 to 24.
![]()
![]()


![]()
![]()
In equation 23, and represent the radii of the combustor before and after the layer of insulation where L is the height of the reactor. A represents the area across which heat transfer occurs. Equations 26 and 27 illustrates how the area is calculated where L is the height of the reactor, already determined.


![]()
![]()
![]()
![]()


Temperatures larger than 60°C cause discomfort in a plant environment for the plant workers. For this reason, the surface wall temperature of the fire clay brick on the sides should be restricted to a maximum of 55 °C.
6 Fluidised Bed Reactor Design for Pyrolysis: Safety
Some safety elements have already been considered in the previous sections of this report, for example, a maximum allowable wall temperature of 55 °C.
A key safety consideration is the absence of oxygen in the pyrolyser as this could lead to an explosion. To combat this issue, a para-magnetic sensor to detect the presence of oxygen is placed in the pyrolyser to issue a warning (3).
Additionally, the entire system should be cleaned with inert gas, such as Nitrogen, to eliminate the presence of oxygen both at the commencement of the process as well as whenever the oxygen sensor issues a warning.
A pressure sensor should be placed in both the combustor and the pyrolyser to detect significant pressure changes as the normal operating pressure of both beds is atmospheric pressure. An anomalous pressure reading could indicate a blockage in either the screw conveyors or the distributor and would require further investigation.
A level sensor should be placed above the total disengagement height to detect the presence of large amounts of particles and to issue a warning that the fluidisation velocity is too high, and that reactor inspection is necessary. Before any inspection, the reactors should be allowed to cool to room temperature. Employee education is vital to ensure a safe process. Personnel working around the fluidised beds should be informed of safety strategies and become aware of all hazards as well as safeguard themselves by wearing PPE.
7 References
- Aho, A, Salmi, T and Murzin, DY (2013), Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals, Elsevier.
- Merckel, R (2014), “Fast and microwave-induced pyrolysis bio-oil from eucalyptus grandis: Possibilities for upgrading,” Department of Chemical Engineering, University of Pretoria.
- Adebayo, K., Coetzee, D., Leher, S. and Viljoen, S, (2019), “Catalytic Pyrolysis of Biomass”, Department of Chemical Engineering, University of Pretoria.
- Bridgwater, T, Meier, D and Radlein, D (1999), “An overview of fast pyrolysis of biomass,” Organic Geochemistry, 30, 1479 – 1493.
- Bridgewater, A (2002), Fast Pyrolysis of Biomass: A Handbook Volume 2, CPL Press, Newbury, UK.
- Huber, G, Iborra, S and Corma, A (2006), “Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering,” Chemical Reviews, 106 (9), 4044 – 4098.
- Zhang, L, Bao, Z, Xia, S, Lu, Q and Walters, K (2018), “Catalytic pyrolysis of biomass and polymer wastes,” Catalysts, 8 (659), 2–45.
- Merckel, R (2019), “The impact of oxygen exothermicity on energy quality of biofuels, and catalytic upgradation,” Department of Chemical Engineering, University of Pretoria.
- Guerrero, R, M. and Millera, A (2005), “Pyrolysis of eucalyptus at different heating rates: studies of char characterisation and oxidative reactivity,” Journal of Analytical and Applied Pyrolysis, 74, 307 – 314.
- Sinha, S, Jhalani, A, Ravi, M and Ray, A (sa), Modelling of Pyrolysis in Wood: A Review, Department of Mechanical Engineering, Indian Institute of Technology, New Delhi.
- Liao, W and Thomas, SC (2019), \Biochar particle size and post-pyrolysis mechanical processing affect soil pH., water retention capacity, and plant performance,” MDPI Soil Systems, 3 (14).
- Swart, S. D., (2012), “Design, Modelling and Construction of a Scalable Dual Fluidised Bed Reactor for the Pyrolysis of Biomass,” Department of Chemical Engineering, University of Pretoria.
- Bamido, A (2018), “Design of A Fluidised Bed Reactor for Biomass Pyrolysis,” Master’s Thesis, University of Cincinnati.
- Du Plessis, B. (2019), “Fluidisation”, University of Pretoria, Dept of Chemical Engineering.
- Wen, C. Y., and Y. H. Yu. “A Generalised Method for Predicting the Minimum Fluidisation Velocity.” Freshwater Biology, Wiley/Blackwell (10.1111), 17 June 2004.
- Vakkilainen, E.K, (2017), Steam Generation from Biomass, Butterworth-Heinemann
- The Thermal Insulation Association of Southern Africa, (2001), “Thermal Insulation Handbook,” Association of Architectural Aluminium Manufacturers of South Africa, Lyttelton.
- org, (2019), “What are the standard sizes of clay bricks?”
About EPCMhttps://epcmholdings.com/about-us/

