1 Introduction
Ammonia is an important and versatile commodity, finding uses in a wide range of Fertilizers, Industrial Chemicals and as a Refrigerant. It is a globally manufactured and traded commodity. The current annual global production of Ammonia is approximately 180 million tonnes. The projected annual growth rate of Ammonia production is estimated between 1 and 1.5 % [2].
About 70% of Ammonia that is produced, is used for fertilisers, while the remainder is used for various industrial applications, such as Plastics, Explosives, and Synthetic Fibres. Ammonia is also injected into Selective Catalytic Reduction (De-NOX) units in fired heaters and power plant boilers, to reduce emissions of Oxides of Nitrogen. The People’s Republic of China leads the world in Ammonia production, contributing about 30% of global output. The other major Ammonia producing nations are Russia (10%), the United States (9%), the Middle East (9%), the European Union (8%) and India (8%). Indonesia, Latin America, Africa, Trinidad and Tobago, also have significant production capacities. Currently there are about 550 Ammonia plants in the world [1].
Ammonia plants are typically part of a larger manufacturing complex producing Urea and other Nitrogenous fertilizers. Hence about 90% of the produced Ammonia is used as feedstock at production sites. The remaining 10% is traded and transported, often covering large distances. The countries and regions that have surplus and dominate the export markets are Russia, Trinidad and Tobago, and the Middle East, representing respectively 24%, 23% and 15% of global Ammonia exports in 2019. The main importers of Ammonia are the European Union, India, and the United States, at 24%, 14% and 13% of global imports [1]. Figure 1 shows historical global trends for Ammonia consumption by the Nitrogenous fertilizer and other industrial sectors.

In the future, as the world transitions to a low Carbon model of industrial development, Ammonia has an important role to play, as a Carbon-free energy source and carrier of Hydrogen.
2 Conventional Ammonia Manufacturing Process
Current processes for Ammonia manufacturing are entirely based on fossil fuels. About 70% of global Ammonia production utilizes Steam Methane Reforming of Hydrocarbons such as Natural gas or Naphtha, to produce Hydrogen needed for Ammonia synthesis. The remaining production comes from Coal gasification, primarily from China, which has abundant Coal reserves.
Ammonia production by the conventional process involves the following steps
- Production of pure Hydrogen from fossil fuels by reforming or gasification, including CO2 removal.
- Addition of Nitrogen either as air or as pure Nitrogen.
- Ammonia synthesis by the Haber-Bosch process, in which the Hydrogen is reacted with Nitrogen from the air to produce Ammonia.
Ammonia (NH3) is synthesized from Hydrogen (H2) and Nitrogen (from the air). Hydrogen is usually produced by the Steam Methane reforming (SMR ) process when Natural gas is the feedstock, though Autothermal reforming (ATR ) can also be used if economics are favourable. In the case of Coal feedstocks, Hydrogen is produced through gasification processes including Partial Oxidation (POX). The conventional Ammonia process from Natural Gas involves primary and secondary reforming steps to produce to produce a mixture of Hydrogen, Carbon Monoxide, Carbon Dioxide, Methane and Water termed “Syngas”, along with Nitrogen required for Ammonia synthesis. This gas mixture is then sent to Shift Converters to oxidize all the Carbon Monoxide to Carbon Dioxide, which is then removed using special solvents. Any residual amounts of Carbon Monoxide and Dioxide are further eliminated in the Methanation step, after which the stream is sent to the Ammonia reactor. Nitrogen (N2) and Hydrogen (H2) react in the presence of an Iron catalyst, to form Ammonia (NH3). The reaction is typically conducted at 400-500 degrees C and 200-300 barg, in a catalyst packed Ammonia reactor. The Ammonia synthesis reaction is:
![]()
Typically, about 18 to 26% conversion of Hydrogen is achieved per pass, through the reactor, hence recycling is necessary. The product gas from the Ammonia reactor is cooled gradually to 12 degrees C, to enable most of the Ammonia to condense out and be recovered. The high-pressure liquid Ammonia is then decompressed to 25 barg, so that unconverted and non- condensable gases are removed and recirculated to mix with fresh feed. Chilled Ammonia at about 15 degrees C can be stored under pressure of about 6 barg or at atmospheric pressure if refrigerated down to -33 degrees C.
Figure 2 is a schematic of the process to manufacture Ammonia from Natural Gas and Air:

3 Decarbonization of Ammonia Manufacture
The overall driver for decarbonization is the global consensus from the Glasgow Climate Pact 2021 (COP26), to achieve Net Zero Carbon Dioxide emissions (NZE) by 2050. Due to its dependence on fossil fuels, Ammonia production is emissions intensive. In the year 2020, Ammonia production accounted for 2% of total final energy consumption globally. Of this energy, 40% was attributable to feedstock energy content and the remainder as fuel energy. Among the fossil fuels, Natural Gas accounts for 70% of the Ammonia industry’s total energy consumption, Coal consumes 26%, Oil about 1% and Electricity the remaining 3%. Direct Carbon Dioxide emissions from global Ammonia production currently amount to 450 million tonnes per annum. To put it in perspective, this is roughly the Carbon footprint of the total energy system emissions of South Africa [1].
In the popular terminology of decarbonization, Ammonia from conventional fossil fuel-based plans is termed Grey Ammonia. When Carbon Capture and Sequestration (CCS) is added to the process scheme (either onsite or through third parties), the Ammonia is termed Blue Ammonia. When the energy sources are non-carbonaceous as in renewable energy, and feedstock is Carbon free, we get Green Ammonia.
Blue Ammonia has an important role to play in accelerating the transition to the Net Zero emission goal, as it can utilize existing manufacturing facilities. Blue Ammonia must be prepared from Blue Hydrogen, which is manufactured by SMR /ATR/POX technologies in combination with CO2 Capture and Sequestration (CCUS). Feasibility of Carbon Dioxide sequestration is location specific, and it requires significant investments, which are drawbacks to implementation.
While Blue Ammonia may be an interim solution, the final objective is to produce Green Ammonia. This would eliminate the use of fossil fuels which are produced through inherently polluting mining or drilling processes.
Table 1 summarizes the energy and Carbon Dioxide emission intensities of Grey and Blue Ammonia:

4 Market Opportunity for Green Ammonia
In addition to progressive decarbonization of Ammonia for fertilizer and industrial applications, there an emerging future for Ammonia as a fuel and energy vector [7]. It is a facilitator for the Hydrogen economy, acting as a chemical medium to store Hydrogen while eliminating the unique safety risks and technological challenges associated with Hydrogen storage and transportation. The concept here is that Ammonia made by electrolysis of Water to Hydrogen and subsequent synthesis, would be transported to Hydrogen consumers as Ammonia, using existing Ammonia infrastructure and supply chains. At the user end, Ammonia would be cracked catalytically to yield Hydrogen and Nitrogen.
Ammonia is well understood by the industry in terms of safety aspects, technologies, and the industry operational practices are standardized across the world. Global market access is ensured by the huge existing Ammonia transportation and storage infrastructure. For example, the United States alone has over ten thousand Ammonia storage sites, connected via a 3000-kilometre-long pipeline network that runs across the country from the Gulf of Mexico to the Mid-West. The European continent boasts of the largest Ammonia pipeline in the world, namely the Tolyatti-Odessa pipeline. This pipeline runs from Russia to Ukraine and has length of 2471 kilometres [1].
The two most promising initiatives that augur well for marketing Green Ammonia as a Carbon neutral energy vector are:
Ship Transportation: The International Maritime organisation (IMO) has mandated that Carbon Dioxide emissions from marine vessels must reduce by 40% of the emission estimated for the year 2008. Further 70 % reduction of Carbon Dioxide emissions from the 2008 base year should be achieved by the year 2050. In response, many companies are working to develop Ammonia-fuelled ship engines. Ammonia as ship engine fuel may create a demand of 450 million tonnes per year if it were to completely replace fossil fuels [7]. Hence this is a huge potential market driven by regulatory pressures. Considering the volumes involved, both Blue and Green Ammonia will play a role, with presumably Green Ammonia being incentivised over Blue.

Power generation: Japan has an ambitious programme to utilize Green Ammonia for electric power generation. The Green Ammonia is likely to be sourced from Middle-East producers. The intent is to replace 20% of Coal used in power station by 2030 [7]. Large Hydrogen fired Gas Turbines already exist in the portfolio of major manufacturers, so Ammonia can be cracked to Hydrogen and used in the power turbine. Additionally, Japanese companies are developing Gas Turbines that can directly use Ammonia as fuel. Mitsubishi Power have announced that they are developing a 40-MW class gas turbine that can directly burn 100% Ammonia [8]. Once this development work succeeds, it would be the world’s first commercialized gas turbine at this scale, to be fuelled by Ammonia [8]. One can expect therefore that the power generation market for Green Ammonia will also develop once countries come under pressure to phase out fossil-fuel based power stations.
5 Overview of Green Ammonia Technologies
The concept underlying Green Ammonia is to reduce its Carbon footprint by utilizing Carbon free Hydrogen and Nitrogen for its synthesis. Hydrogen is produced by the electrolysis of demineralized Water. Nitrogen is produced by using Air separation units which could be Molecular Sieve (PSA) or Membrane type for the smaller sizes and cryogenic separation at large scale. Synthesis of Ammonia is performed by the well-established Haber Bosch process, though some innovations have been implemented for mini-Ammonia reactors.
Figure 4 illustrates the main features of the Green Ammonia process:
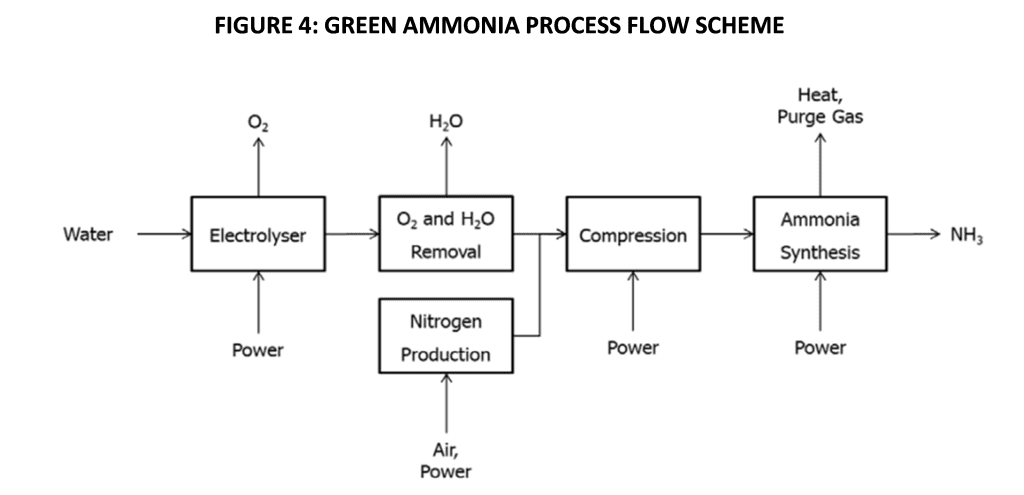
A Green Ammonia Plant consists of following units:
- Hydrogen Production
- Nitrogen Production
- Ammonia Production
- Hydrogen Storage
- Ammonia Storage
- Balance of Plant (utilities and infrastructure)
Salient functional and technical aspects of each unit are explained in the following paragraphs.
Hydrogen Production
The function of this unit is to produce Pure Hydrogen at the required rate, by the electrolysis of Water. An electrolysis cell uses direct current electric supply to split Water into Hydrogen and Oxygen. This is performed in electrolysis cells comprising several individual cells arranged in one or more stacks with reactant water flowing through the cells via input and output conduits formed within the stack structure. The typical potential difference across the electrodes in commercial cells is around 1.8-2.0 V. The reaction rate improves at higher temperatures due to reduced electrolyte resistance and the driving voltage reduces. Allowance is made in the design for performance degradation over time.
Roughly ten litres of Demineralized Water are utilized for every kilogram of Hydrogen produced. Demineralized Water is pumped into the electrolyser stack cell, where it is distributed to individual cells for splitting into Hydrogen and Oxygen. From a safety perspective, isolation of Hydrogen and Oxygen streams is crucial to avoid potentially explosive conditions. This is ensured in design of the cells as well as in the overall piping system design. After knocking out entrained water, the Hydrogen stream is purified in a deoxidizing tower and then dried before being compressed and sent to storage. Generally, the Oxygen is vented safely to atmosphere via a regulating valve, unless the project specifically seeks to monetize the Oxygen stream. Figure 5 shows a typical Hydrogen Electrolyser unit.

Nitrogen Production:
Due to the relatively small scale of Green Ammonia plants, the most popular technology for Air separation is Pressure Swing Adsorption (PSA), which delivers high purity Nitrogen (99.9995%) at competitive cost. In contrast, Membrane type Nitrogen units, which use hollow fibre membranes, achieve about 99.5% Nitrogen purity, increasing downstream purification costs. In a typical PSA plant, filtered and dried compressed air at about 8 barg, enters the PSA Nitrogen towers. These towers are packed with Carbon Molecular Sieves that preferentially remove Oxygen, allowing pure Nitrogen to pass through. Upon saturation the Adsorbent bed has to be regenerated, which done by depressurizing, vent, and purge. Hence a PSA unit will have at least two towers. Typically, gas flows upward through the adsorbent bed, being dehydrated at the bottom of the adsorption tower, and then enters the Adsorbent bed. Pure Nitrogen leaves at the top of the tower and enters a Nitrogen storage tank. When one adsorption tower is performing adsorption, the other adsorption tower would be under regeneration. The two towers alternately perform adsorption and regeneration to achieve continuous flow of pure Nitrogen. The Nitrogen in the storage tank is sent to a Deoxidizing tower where Oxygen present as impurity reacts with Hydrogen to form Water in the presence of a catalyst. The Oxygen content in Nitrogen leaving the system is controlled to be ≤1ppm. The gas is then cooled to 40°C by a water cooler and sent to a drier which typically works on the Thermal Swing Adsorption (TSA) principle. Here the moisture content is reduced to ≤5ppm after which Nitrogen is sent for Ammonia synthesis.
Ammonia Production :
Purified Nitrogen and Hydrogen are mixed in a ratio of about 1:3 in a mixing buffer tank along with recycle gases from the Ammonia loop. The gases are compressed to a high pressure depending on the Synthesis Reactor technology and proprietary catalyst offered by various vendors. Similar to the conventional process, only partial conversion is achieved on each pass through the reactor and unconverted gases have to be recycled. The product gases are cooled in several stages such as air-cooler, water cooler, cold exchanger and Ammonia chiller in sequence. Unlike conventional Ammonia plants, there is no need to produce Steam from waste heat, due to the small size and lower temperatures at which Green Ammonia plants operate. After the temperature is lowered in the Chiller, condensed Ammonia is removed in the separator and pumped to the Ammonia Storage tank after decompression. Flash gas after decompression is a small quantity which is vented at a safe height. The recycle gas from the separator enters the cold exchanger and then the recycle loop via the Syngas compressor .
Liquid ammonia storage :
Ammonia can be stored in pressurized bullets or spheres at about 8 barg. It can also be stored in atmospheric cryogenic tanks at -33°C. The decision will be project and site specific. The inventory requirements are based on assessment of market dynamics and any offtake agreements in place for the product.
Hydrogen Storage :
Storage of Hydrogen requires compression to high pressures. Since the Ammonia synthesis reactor operates in a range of 200 to 300 barg, a typical choice would be to store the Hydrogen at about 300 barg. However, innovations in mini-Ammonia reactor design are available and many vendors are offering lower operating pressures and temperatures. This would reduce the Hydrogen compression costs.
Balance Of Plant
The following is a detailed list of balance of plant items for a large Green Ammonia project which utilises Alkaline Water Electrolysers and incorporates Oxygen recovery.
6 Project considerations
Production capacity:
Economy of scale is important to establish commercial viability and deliver Ammonia to the market at competitive prices. Conventional Ammonia plants based on fossil fuels generally range from 600 TPD to 3300 TPD [1].

In contrast, Green Ammonia projects depending on wind, solar energy or grid power are currently being developed as on a much smaller scale. Typical green Ammonia plants have capacities from 1 TPD to 60 TPD. These are therefore termed mini-Ammonia plants and several vendors around the world are now offering skid based mini-Ammonia plants.
None of these mini-Ammonia projects are viable without access to subsidized finance. The business rationale driving investment is not profit but usually other considerations such as ESG pressure from stakeholders, availability of tax credits and other incentives provided by governments. Concessional power supply is a key aspect that will determine viability of electrolyser-based projects.
It may be noted that the scalability of green Ammonia is largely influenced by electrolyser capabilities and green power. Capacity need not be a constraint if government policy drives the investment. Examples of recent world scale electrolyser facilities are the 150MW Baofeng Green Hydrogen plant in China which is reported to be operational. Another 260 MW alkaline electrolyser facility is being established for Green Hydrogen in the Xinjiang region by Sinopec [11].
Availability of Raw Materials
Conventional Ammonia plants use fossil fuels both as feedstock and fuel. Hence, locations of these Ammonia plants are tied to supplies of competitively priced fossil fuels. Availability of low-cost natural gas spurred the establishment of gas-based Ammonia plants in the United States, Middle East, and Russia. Similarly, China’s abundant coal reserves explains its focus on Coal-based Ammonia. which accounts for around 85% of its production. Countries utilising Naphtha-based reforming have located their Ammonia plants near Crude Oil refineries
Unlike conventional, plants Green Ammonia requires fresh water and air as raw materials. This changes the plant location paradigm. Production of pure Hydrogen via electrolysis requires high quality Demineralized Water. Presence of impurities affects the life span and efficiency of electrolysers. It may be noted that saline Water electrolysis is not suitable for large scale Hydrogen manufacture due to Chlorine byproducts.
Demineralized Water requirement for electrolysis about is 10 kg per kg of Hydrogen produced. Apart from that, good quality cooling water is required for electrolyser stack cooling as well as for several equipment in the Ammonia plant and utilities areas. Abundant treated water supply at competitive rates is a key consideration for locating Green Ammonia projects.
Availability of stable supply of green electricity
In theory Electricity can be produced from virtually any non-fossil energy source, including hydro, geothermal, nuclear power, and variable renewable energy (VRE) sources such as solar PV and wind. Hydropower for production of Hydrogen by electrolysis for Ammonia synthesis was in vogue through most of the early twentieth century. In the 1960s and 1970s, wider availability of natural gas at competitive prices led to increasing use of Natural Gas-based steam reforming for Hydrogen production, due to its lower overall production costs. Most of the electrolysis-based plants shut down over time. Following the closure of a plant in Egypt in 2019, only one known hydropower electrolysis-based Ammonia plant remains in the world: a small facility in Peru using a 25 MW electrolyser [1]
All the Green Ammonia projects being pursued today are either based on wind energy or solar or use a hybrid configuration including renewable and grid power hybrids. The renewable power generation facility need not be part of the Ammonia project. It is recognized that that the ideal sites for renewable power production and those for Ammonia production may not coincide. Most governments are permitting Green Ammonia plants to stay grid connected while purchasing renewable power from a remotely connected supplier who is connected to the grid. Wheeling charges may be applicable or even waived in cases where government is promoting green energy. There must be a power purchase agreement between Ammonia producers and renewable energy producers. This arrangement allows the Green Ammonia facility to benefit from stability of the grid, increase the plant availability and reliability significantly.
In case grid connectivity is not possible due to limitations of the grid or remote project locations, then the renewable power source must be in proximity to the Hydrogen and Ammonia production facility. They can be part of the same project.
Wind and solar energy are inherently intermittent and vary in intensity. This affects electrolyser performance. Ammonia, being a high temperature catalytic process needs stable operating conditions to function properly.

It is therefore a challenge for green Ammonia projects to deal with this variability. Most of the large-scale electrolyser projects recently completed or currently underway are tapping power from the grid.
Electrolysers that depend entirely on variable renewable energy sources need to be oversized. For example, if renewable energy available only for eight hours a day, then the electrolyser needs to produce the required daily Hydrogen quantity in 8 hours instead of 24 hours. This means the electrolyser will be three time larger than it would be if it were taking 100% grid supply, which has 24×7 availability. In addition to higher CAPEX for electrolysers, it becomes necessary to have increased Hydrogen storage, to allow the Ammonia plant to function continuously even in the period when there is no power generation from VRE sources.
Cost of electricity
Based on typical electrolyser power consumption of 55 kwh per kg of H2, power consumption for Green Ammonia constitutes 85 to 90%of the OPEX for grid connected electrolysers. Hence cost of electricity determines viability of Green Ammonia projects. Of course, the cost of grid electricity is country dependent. A wide range of variation in pricing has been noted in national average electricity prices for industry. A tariff range of USD 22-240 per MWh has been noted and the global average is around USD 100 per MWh [1].
Choice of Electrolyser technology
From commercial perspective, the choice is between two technologies, namely
- Alkaline Water Electrolyser (AWE)
- Polymer Electrolyte Membrane Cell (PEMC)
These are the only two electrolysis technologies that are mature and available at Megawatt scale. Salient features of each are as follows:
- Alkaline Water Electrolyser:
Alkaline Water electrolysers use an alkaline solution (of sodium or potassium hydroxide) that acts as the electrolyte. These electrolysers have been commercially available for over a hundred years. They can be configured as unipolar or bipolar (filter press) designs. In the unipolar design, the electrodes are submerged in the alkaline electrolyte, inside a tank. The electrolyte is a 20%-30% solution of potassium hydroxide in pure water. Each cell is connected in parallel. These electrolysers are simple to manufacture and repair, but they have lower current densities and temperatures then PEMC. Current designs can operate at high pressure outputs, up to 41MPa (6,000 psig).

Figure 6: schematic of an Alkaline Water Elecrolyser
Figure 6: schematic of an Alkaline Water Elecrolyser
PEM electrolysers use a solid specialty plastic material as electrolyte instead of an alkaline (KOH) electrolyte. General Electric created the first PEM electrolysers in 1966, due to the development of perfluorinated ion-exchange membranes NafionTM by DuPont. However newer developments have occurred, better membranes are available. Protons produced by the electrolysis of water migrate through the polymer electrolyte membrane and combine at the anode with electrons to form Hydrogen.
PEM electrolysers use expensive materials like Platinum, Palladium, Iridium and Rhodium. Deionized water with a high degree of purity is needed to avoid damage to the electrodes. The membrane and noble metals for the electrocatalyst, make the PEM electrolyser more expensive than other kinds.


Figure 7: Schematic of PEMC electrolyser
- Comparison Of technologies
Alkaline Water electrolysis technology today is extremely efficient, reliable, and cost-effective. The technology is mature, and the safety aspects are well known. Alkaline electrolysers of up to 135 MW were known nearly 100 years ago. A 135 MW AWE in the year 1927 could produce about 30,000 Nm3/h of Hydrogen (2.67 tonnes per hour). This corresponds to Ammonia production capacity of about 15 tonnes per hours. Figure 8 is a photograph of these old plants.

Figure 8: Historical Large scale Alkaline Water Eelectrolysers (Source: NEL Brochure)
PEMC on the other hand is available commercially only at a much smaller scale with successful demonstration plants utilizing 5 MW electrolysers. An electrolyser of 5 MW capacity can produce about 1000 Nm3/h of Hydrogen (90 kg/h). This translates to a theoretical Ammonia production of 510 kg/h.

Figure 9: Largest operating PEM based Hydrogen plant (Source: Siemens Brochure)
Any comparison between the two technologies is therefore meaningful only for electrolyser capacities upto 5 MW, corresponding to mini-Ammonia plants of about 12 tonnes per day of Ammonia.
Figure 9, reproduced from a report by CEMAC/NREL, provides a comparison between key performance parameters of AWE and PEMC electrolysers:

Figure 9: Key technology parameters compared for Alkaline and PEM cells (source: CEMAC presentation at Fuel Cell Seminar and Energy Expo, Aug 2017 by CEMAC/NREL)
Ultimately the choice of PEM vs alkaline depends on the application and parameters at play. They both have their advantages for different applications, and both technologies are improving rapidly. Scale, input power characteristics, electricity cost, and rate of technology development will be deciding factors in future.
In the near term, large alkaline plants will have an advantage in industrial settings where large grey Hydrogen producers are shifting to green Hydrogen such as fertilizers and Refineries. PEM on the other hand will be preferred for decentralised production of Hydrogen or where very compact plants are required.
Utilization of Oxygen
Oxygen is valuable product, and this is one of the considerations when developing a Green Ammonia project. Oxygen is generated in the Electrolyser as well as in the air separation plant
CAPEX and OPEX
Electrolyser costs and Power costs are two major considerations for CAPEX and OPEX respectively. This excludes the CAPEX for solar or wind farm, which is assumed to be by others.
A knowledge sharing report has been published by QNP Australia on their proposed plant to manufacture 3,500 Tonnes/Year of Green Hydrogen and 20,000 tonnes/Year of Green Ammonia [4]. The key project parameters are:
- Water (~75 Million litres/year ) from a nearby river.
- Renewable energy procured through a Power Purchase Agreement (PPA). A transmission network use of system (TUOS) charge is applicable.
- An alkaline electrolyser of 30 MW consuming about 208 GWh of electricity to produce 3,500 Tonnes/year of Hydrogen.
- On-site Hydrogen storage to ensure Ammonia plant functions continuously.
- An Ammonia synthesis plant of 20,000 Tonnes/year
- a new 132 kV transmission line of 5 km length from the local sub-station to the QNP plant.
The estimated CAPEX for the project is 150 to 200 million Australian dollars (AUD). Final break-up of estimated cost is indicated in the chart (Figure10).

The estimated OPEX is in the range of 10 to 15 million AUD per annum. The electricity cost is about 45 AUD per MWh.
The breakup of Operating and maintenance expense is shown in Figure 11.

7 References
- International Energy Agency (IEA), 2021. Ammonia Technology Roadmap CC BY-NC 3.0 IGO.
- Power to Ammonia : Feasibility study for the value chains and business cases to produce CO2 -free ammonia suitable for various market applications, ISPT, 2017
- ‘Green Ammonia ‘ prices double that of regular supplies; news provided by Argus media, June24,2021.
- QNP Green Ammonia Project Feasibility Study Knowledge Sharing Report, June 2020, Queensland Nitrates Pty Ltd (QNP).
- Preliminary Feasibility Study Of The Establishment Of A Chemical Fertilizer Plant In Nepal, by Prashant Luitel, Investment Board of Nepal, Government of Nepal, 2014.
- From MEGA-ammonia to mini-AMMONIA, Presentation at the 7th NH3 Fuel Conference Detroit 1 26-9-2010 till 29-9-2010, By: J.P.Vrijenhoef, Proton Ventures BV The Netherlands
- Toward Green and Blue Ammonia, by CASALE SA, May 12,2021; Toward Green and Blue Ammonia (energy.gov)
- Mitsubishi Power Developing 100% Ammonia-Capable Gas Turbine (powermag.com)
- 9. CEMAC presentation at Fuel Cell Seminar and Energy Expo, Aug 2017 by CEMAC/NREL).
- 10. NFuel conference Detroit rev 1 [Compatibiliteitsmodus] (ammoniaenergy.org)
- 11. Record breaker | World’s largest green hydrogen project, with 150MW electrolyser, brought on line in China | Recharge (rechargenews.com)
- Green Ammonia and Hydrogen at Scale, August 2019, SIEMENS,www.seimens.com.

 To all knowledge
To all knowledge