Summary
Meeting the growing energy demands of the modern world has become a pressing challenge. The availability of conventional fossil fuels is becoming increasingly limited. Still, there are growing concerns about the environmental impact of fossil fuel emissions. Biodiesel is among the most prominent biofuels due to its environmental and technical advantages over petroleum diesel. However, biodiesel’s production process is more expensive than traditional diesel due to the high cost of raw materials. In most countries, biodiesel production mainly relies on animal fat and waste frying oil as the primary raw materials. Nonetheless, it is worth noting that blends of canola straight oil (CSO) and cotton seed biodiesel (CSB) with petroleum diesel have great potential as fuels for future biodiesel engines. These fuels are yet to be fully utilized due to a lack of awareness of their benefits and limited research on their production processes. However, their potential for use as alternative and sustainable fuels is undeniable, especially considering limited reserves of conventional fuels and the urgent need to mitigate their environmental impact. As such, continued investment in research and development of these fuels is essential for a more sustainable energy future.
Historical Background
An analysis of the history reveals that the term “biodiesel” was first used in 1988. In 1900, Rudolf Diesel first demonstrated its use at a fair in Paris using peanut oil as a source of biodiesel. In later years, the less expensive fossil fuel-based diesel fuel, which was also heavily subsidized by governments and extensively used by consumers, caused the use of vegetable-based diesel fuel to be discontinued until the 1920s.
By the 1940s, countries that produced crops took significant steps to become self-sufficient in energy. This is because they already knew that vegetable oils could be used as fuel. For instance, Brazil forbade the export of cottonseed oil during World War II while still using it as a diesel substitute. A lack of fossil fuels and worries about energy security in the second half of the 20th century rekindled interest in creating fuels based on vegetable oils as an alternative energy source [1, 2].
However, the first generation of biodiesel was unsuitable for diesel engines. Biodiesel had a higher viscosity than petroleum-based diesel, which caused problems with deposits and engine part damage, and poor atomization within the engine. Several procedures, including pyrolysis, microemulsification, and blending, were created to enhance the quality of biodiesel [3].
Many successful studies on using biodiesel as a fuel for diesel engines were carried out in the 1980s, and the findings were widely disseminated. The first biodiesel plant on an industrial scale was built in 1989 by an Austrian business. Despite this advancement, substantial subsidies for fuels made from petroleum have hampered biodiesel development.
At the close of the 20th century, attention returned to the creation of biodiesel due to escalating environmental concerns. The development of standards like ASTM D6751 and EN 14214 has resulted from the recent trend in many nations to prioritize the use of biodiesel blends in place of conventional diesel. Many gas stations in the United States and Europe now sell biodiesel blends. Biodiesel’s significance in the world’s energy system is growing due to ongoing research and development [4].
Introduction
The Industrial Revolution, which occurred in the early 19th century, ushered in an era of economic growth and improved living standards. This led to an increased demand for energy, with fossil fuels emerging as the primary global energy source. Today, approximately 88% of the world’s energy is generated from conventional fossil fuels, and the transportation sector relies almost entirely on petroleum-based fuels. However, limited availability and environmental concerns have prompted scientists to explore alternative energy sources, including renewable and inexhaustible options like biomass, wind, solar, nuclear, geothermal, and biofuels [5, 6].
Manufacturing and using sustainable, environmentally safe fuels like biofuels have received more attention in recent years. The technical and environmental advantages of biodiesel over petroleum-based diesel set it apart from the other biofuels on the market. The ASTM defines biodiesel as a fuel made from vegetable oil or animal fat, primarily monoalkyl esters of long-chain fatty acids. Most of the animal fats and vegetable oils used to make biodiesel are triacylglycerols (TAGs), which are chemically bound chains of fatty acids.
Biodiesel is a sustainable energy source, as it can be produced from both edible and non-edible vegetable oils and offers several advantages over traditional diesel fuel. When used in automobiles, biodiesel generates less harmful emissions such as carbon monoxide, hydrocarbons, and particulate matter, making it a more environmentally friendly choice.
Additionally, biodiesel has a higher cetane number and better lubricating properties, which can improve engine performance and reduce wear and tear. It also has a relatively high flashpoint of around 150 °C, making it safer to store and transport than conventional diesel fuel [7]. Biodiesel can be produced on a small or large scale, depending on the requirements, and can be made in various locations, from urban to rural areas.
CSB is a promising alternative to conventional fossil fuels because it is renewable and sustainable. It is produced by extracting oil from cotton seeds, which involves pressing and crushing the seeds to obtain the oil. This raw oil is then converted into biodiesel using a chemical process broadly termed as transesterification. In conclusion, even though there are currently few applications for CSB, there are still some instances of its use around the globe.
Table 1. Some of the key properties of cottonseed oil [8]
| Property | Units | Value |
| Calorific value | MJ / kg | ~ 40 |
| Density at 288 K | kg / m3 | 920 – 925 |
| Flash point | oC | ~ 234 |
| Pour point | oC | -15 – 7 |
| Kinematic viscosity at 300 K | mm2/s | ~ 40 |
| Carbon residue | % w/w | 0.24 |
| Cetane number | – | ~ 47 |
| Iodine value | mg I/g | 90 – 120 |
While the global biodiesel market is growing, the market size for CSB is limited due to various factors. The increasing demand for cottonseed oil in the food and beverage industry is driving the growth of the cottonseed oil market. The drawbacks of using CSB, such as its limited supply and potential NOx emissions, highlight the need for additional research and funding to remove these obstacles and encourage its wider adoption as a renewable and sustainable fuel source.
Cottonseed Biodiesel (CSB)
CSB is a renewable, clean-burning fuel produced from the oil extracted from the seeds of cotton plants. Cottonseed oil is a by-product of cotton production and is commonly used for food and industrial purposes. It has several properties and characteristics that make it a viable alternative to petroleum-based diesel fuel.
One of the main advantages is its high cetane number, which measures the fuel’s ignition quality. Biodiesel produced from cottonseed oil typically has a cetane number between 58 and 66, which is higher than that of petroleum diesel. This means cottonseed biodiesel has a shorter ignition delay, leading to better combustion and lower emissions.
Another advantage of CSB is its low sulfur content, which reduces emissions of sulfur oxides (SOx), a significant contributor to air pollution. CSB also has a higher flash point and lower volatility than petroleum diesel, improving safety and reducing emissions of volatile organic compounds. CSB can be used in existing diesel engines without the need for modifications or blending with petroleum diesel. It can also be blended with petroleum diesel in varying proportions, depending on the desired fuel properties and emissions requirements.
In terms of applications, CSB can be used in a wide range of diesel engines, including those in transportation, agriculture, and construction. It can also be used in stationary engines for electricity generation and heating. CSB can be stored and transported like petroleum diesel, and it has a shelf life of at least six months with proper storage. With further research and development, cottonseed biodiesel has the potential to play an important role in the transition to a more sustainable energy system [9, 10].
Production Methods
Biodiesel is produced by refining raw vegetable oils such as cottonseed oil. Raw vegetable oils are not suitable for direct use in diesel engines due to their high viscosity, polyunsaturated character, and low volatility. These properties can cause engine problems such as clogging and reduced fuel efficiency. To make them fit for use in diesel engines, the oils must undergo a refining process.
Micro emulsions and direct mixing are the most commonly used methods for incorporating vegetable oil into diesel engines. By using these methods, the oil viscosity is effectively reduced without the use of chemicals. They do not, however, address the issue of lube pollution and carbon deposits, which can cause engine damage and shorten the engine’s lifespan.
To produce high-quality CSB, raw vegetable oil is subjected to a chemical process called transesterification. During this process, the vegetable oil is combined with an alcohol, typically methanol, and a catalyst, such as sodium hydroxide or potassium hydroxide, to produce biodiesel and glycerol. The resulting biodiesel is a clean-burning fuel that can be used in diesel engines without the risk of engine damage [11, 12].
The quality of CSB is often dependent on a variety of factors mentioned in the follows,
- Temperature scheme of the synthesis reaction
- Percentage composition of vegetable oil and alcohol
- Type and amount of catalyst for the synthesis
- The mixing or stirring system
- The type and purity of raw oil used
A general process scheme of producing the alkyl fatty acids of biodiesel by using vegetable oils is given in the follows [4, 13],
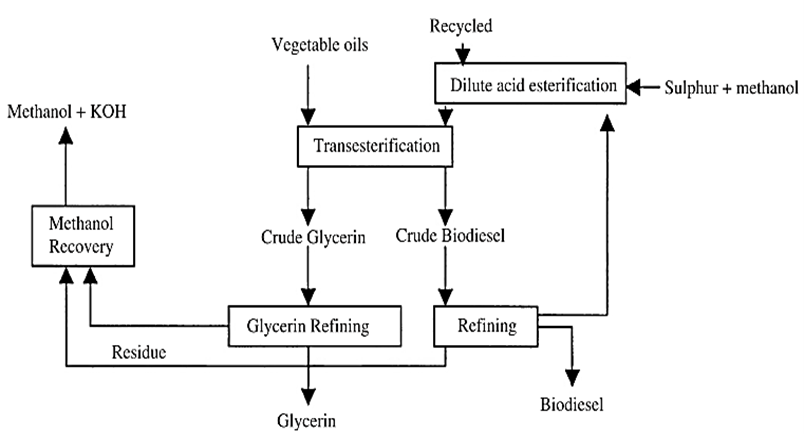
Figure 1. Process scheme of TAG conversion to yield alkyl fatty acids [14]
Transesterification Method
One commonly used method for producing CSB involves a transesterification process. In this, monohydric alcohol and TAG are combined at elevated temperatures in the presence of a catalyst. The resultant products are fatty acid alkyl esters and glycerol, as shown below,
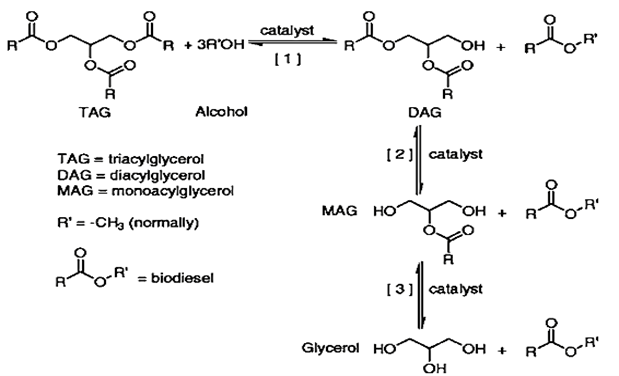
Figure 2. Transesterification method that produces fatty acid alkyl esters [6]
The overall transesterification process can be broken down into three intermediate steps. These steps are reversible reactions, and the first involves converting triglycerides to diglycerides. The next stage is the production of monoglycerides from diglycerides, and the final step is the conversion of monoglycerides glycerol. A similar reaction scheme is also involved in the production of esters. Even though a stoichiometric ratio between oil and alcohol exists but typically, an excess quantity of alcohol is used to improve the production of desired products. The three steps are shown in detail in the following figure, in which a triglyceride of oil is reacted with methanol.
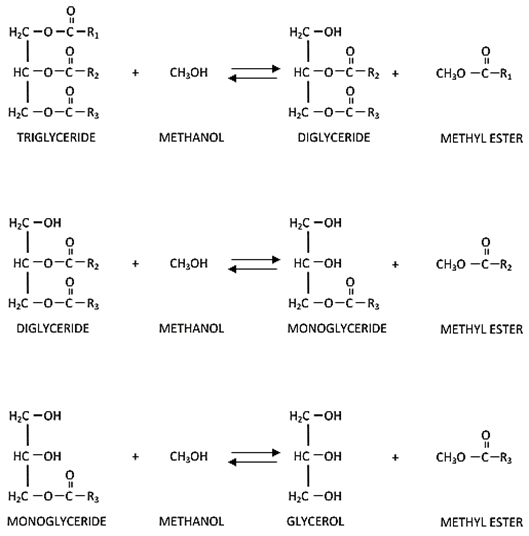
Figure 3. The three individual steps of the transesterification reaction [15]
Other than methanol, alcohol ranging from ethanol to butanol and amyl alcohol are the potential candidates for transesterification processes. Most frequently, methyl or ethyl alcohol are used because of their low cost and physical or chemical advantages. Methanol is the most preferred one because it can easily dissolve sodium hydroxide and can react with triglycerides at a faster pace.
A transesterification reaction also needs a catalyst for shifting the production equilibrium towards the favorable side. Therefore, several catalysts such as acids, alkalis, and enzymes are typically used. The process schemes when using these different types of catalysts are further elaborated in the subsequent sections [16-18].
Alkali-based Catalysis
In this type of catalysis, two commonly used bases are combined with the raw material. These can be used with either ethanol, methanol, refined, or waste frying oil. To obtain a better efficiency in the production process, alkoxy is first produced before the transesterification reaction. The most commonly used ratio of alcohol to oil is 6:1 in the presence of an alkali catalyst [14].
R – CH2OH + NaOH à H2O + R – CH2ONa
Various oils range from edible oils (soybean, canola, peanut) to non-edible oils (cottonseed, rapeseed, Jatropha). The selection of raw oil materials is based on availability and low price in the market. The amount of catalyst used in the reaction can vary around 1% w/w, and percentages as low as 0.005% have also been reported in the literature.
Production is usually carried out at a temperature of 60°C, but it’s much more dependent on the type of catalyst used for the transesterification reaction. Usually, the reaction temperature range is from room to temperature as high as 120 °C. Compared to other acid-catalyzed processes, alkali-based catalysis provides much more efficiency and less problem of corrosion [11, 19].
Acid-based Catalysis
This transesterification process involves using acid-based catalysts such as sulfuric acid and sulphonic acid [20]. The acid-catalyzed transesterification process is slow when compared with the alkaline-catalyzed reaction, but the product yield is considerably high in this reaction. The time durations required for different alcohols in acid catalyzed transesterification reactions are listed as follows,
- When using methanol, the required temperature is 65°C and time is nearly 50 hours for 99% conversion
- With ethanol, for the exact conversion, the time required could be 18 hours at a temperature of 70°C
- With butanol, for the same conversion, three hours are commonly required with a temperature of 117°C.
An overall excessive concentration of alcohol positively shifts the reaction towards the desired products, but under such conditions, the recovery of glycerol would become difficult in this case. The amount of catalyst used for the process is usually around one-mole percent, and the general temperature range is 50 °C to 80 °C. This process is beneficial for all samples with high content of free fatty acids [19, 21].
Enzyme-based Catalysis
Such a type of catalysis is usually preferred because of the biocompatibility and environmental acceptability of the transesterification reaction. The enzymes usually used are named lipases, and they are fully used for conventional processes such as acidolysis and alcoholysis. Such a type of catalysis is usually preferred because of several advantages compared to other processes [14]. However, some of the disadvantages are also present, which are discussed here.
When transesterification is conducted using enzymes, separating the desired product is relatively easy. In addition, due to thermal stability and lipase immobilization, higher catalyst concentrations could be used, and the process could be continued for a longer duration. However, some initial activity could be lost due to the volume of the oil molecule, and the process economics are usually inferior when using biocatalysts [22].
Other Production Methods
Some of the modern production methods are further discussed in this section. A number of methods have been reported in the literature, which includes,
- Supercritical Methanol Transesterification [23]
- Production of Biodiesel Through the BIOX Cosolvent Method [23]
- Algae-Based Biodiesel Production [24]
- Supercritical Methanol Transesterification by Using Catalysis [23]
- Transesterification via Radio Frequency Microwaves [25]
- Pyrolysis
The first two above-listed methods are essential and are discussed in detail in this section.
Supercritical Methanol Transesterification
The conventional transesterification process involves using vegetable oils or animal fats to produce biodiesel. However, the content of fatty acids and water can negatively impact the process through soap production, consumption, and reduction in catalyst effectiveness.
On the other hand, the transesterification process involving acid or base catalysis is much more efficient. However, any such process requires an extended timeframe and complex separation procedures for separating the product. Considering these drawbacks, recently, a new transesterification process involving supercritical methanol in the absence of catalysts was developed.
This process is expected to solve the issue of product separation from the raw materials by forming a single phase. This single-phase formation results due to the low dielectric constant of supercritical methanol. The process possesses several advantages over the conventional catalyzed transesterification processes. These are listed as follows,
- The separation and purification of products are much simpler and cost-effective
- It is essentially a non-catalytic process
- The reaction time is much lower
- The process is considered more environmentally friendly
However, the reaction conditions for this process are much more severe as a temperature of nearly 600K and a pressure of around 50 MPa is required. An illustration of a two-stage continuous production process is given in the figure. During the process, methanol is kept in a supercritical state at around 440K and a pressure of 10MPa. Under such supercritical conditions, the formation of methyl esters from the fatty acids is much more rapid [23].
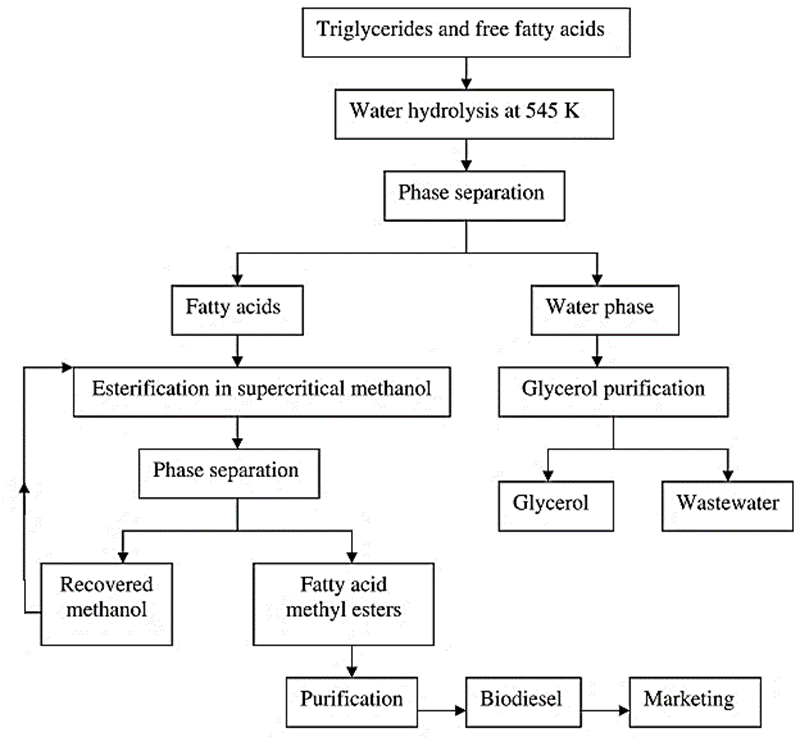
Figure 4. Process Scheme of supercritical methanol transesterification [23]
BIOX Cosolvent Production Method
This method was developed by a professor at the University of Toronto named David Boocock. He presented a unique way of generating a one-phase system using inert cosolvents (such as tetrahydrofuran). The unique feature of this process is the reaction time and conversion. According to literature reports, the reaction time is seconds under ambient temperature, and more than 99% of product conversions are achieved. This process takes place in two steps such as follows,
- The first step involves the conversion of free fatty acids through acid esterification.
- The next step involves the conversion of triglycerides told transesterification by adding cosolvent. This step occurs in two stages under ambient conditions in a single-phase correction system.
After the completion of the reaction, the cosolvent can be recycled and continuously reused within the process. This process offers several advantages, such as the availability of reclaimable inert cosolvents and reaction time in seconds under ambient conditions of temperature and pressure. This process can also use waste cooking oil and animal fats to produce good-quality biodiesel [23, 26, 27].
Fuel for vehicles
Biodiesel made from cottonseed has properties similar to conventional fuels, and typically, it can be used either pure or in a blend with petroleum diesel. Diesel engines can run on biodiesel blends with little to no modification. Before using biodiesel as a vehicle fuel, however, there are some things to consider [28].
According to the literature, conventional petroleum diesel’s heating value is typically about 9% higher than biodiesel’s. If an engine running on biodiesel has the same efficiency as one running on petroleum diesel, it will need more biodiesel to generate the same amount of power. On the other hand, the difference in engine performance efficiencies between the two fuels is almost negligible, but biodiesel consumption is relatively higher [4].
The performance of traditional diesel engines can be impacted in several ways by using biodiesel as a vehicle fuel. A number of variables, including increased lubrication, deposit formation, component degradation, and filter clogging, may impact engine performance. Glycerol content, cold flow behavior, degradation phenomena, and other impurities impact these problems. However, there is still a dearth of research on the long-term performance traits of diesel engines using biodiesel [28].
Several studies have shown that using biodiesel can significantly reduce carbon dioxide emissions (up to 23 percent) and lower chemical emissions after conducting a life-cycle and Well-to-Wheel analysis of carbon dioxide emissions. Due to the variability of the pollutants’ sources and engine parameters like speed, load, ambient conditions, and running time, it can be difficult to pinpoint specific pollutants that biodiesel can reduce [29].
Availability and price are the two main factors to consider when selecting biodiesel, followed by how well it performs in diesel engines. When determining the suitability of a particular biodiesel, performance indicators like brake power/torque, brake thermal efficiency, and brake-specific fuel consumption are frequently considered [30].
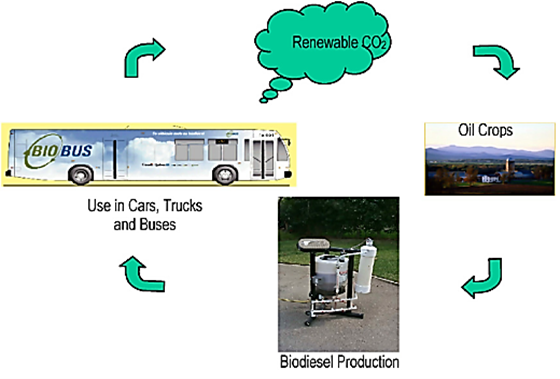
Figure 5. The carbon dioxide life cycle with biodiesel as vehicular fuel [31]
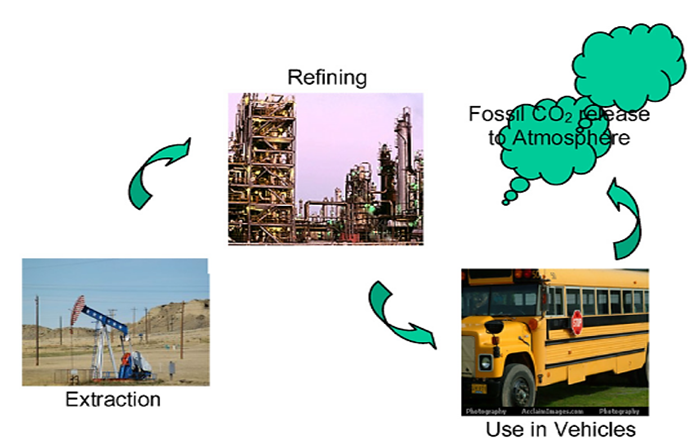
Figure 6. The carbon dioxide life cycle with petroleum diesel as vehicular fuel [31]
Limitations of CSB & Market Trends
Recent studies have shown that cottonseed biodiesel (CSB) can significantly reduce particulate matter, carbon monoxide, and hydrocarbon emissions in diesel engines, thereby reducing air pollution and improving air quality. These studies have also revealed that the use of CSB can lead to a decrease in greenhouse gas emissions and contribute to mitigating climate change. However, using CSB can lead to increased nitrogen oxide emissions, which harm human health and the environment. The literature suggests that further research is still needed to develop methods to control NOx emissions with cotton seed biodiesel blends [32].
CSB is a reasonably specialized biofuel, and consumption is constrained for various reasons. The United States Department of Agriculture (USDA) reported in 2020 that the CSB production was ~ 5.4 million metric tons worldwide, with China, India, Pakistan, and the United States accounting for most of that production. However, only a small portion of this production was used to make biodiesel [33, 34].
The insufficient supply of cotton seeds for biodiesel production is one of the main obstacles to the widespread use of CSB. The availability of cotton seeds for biodiesel production may be constrained because they are also used for animal feed and human consumption. Additionally, CSB production costs are relatively high compared to those of other biofuels, which may make it less commercially viable for some producers.
Despite these limitations, some noteworthy uses for CSB remain. As an illustration, the Indian government has set a goal of blending 5% biodiesel into diesel by 2030, with a sizable proportion of the biodiesel anticipated to be produced from cotton seeds. In West Texas, the United States, a biodiesel plant based on cottonseed oil was established in 2018 with a 40-million-gallon annual capacity [34, 35].
According to a report published by MarketsandMarkets, the global biodiesel market was valued at USD 21.54 billion in 2020 and is projected to reach USD 25.59 billion by 2025, growing at a CAGR of 3.5% during the forecast period (2020-2025) [36]. The report also states that the Asia Pacific region accounted for the largest share of the biodiesel market in 2020 due to the growing demand for biodiesel in countries such as India, China, and Japan.
According to a Global Market Insights report, the global cottonseed oil market was valued at USD 830 million in 2020 and is projected to reach USD 1.2 billion by 2027, growing at a CAGR of 4.7% from 2021 to 2027 [37]. The report also states that the increasing demand for cottonseed oil in the food and beverage industry is driving the growth of the cottonseed oil market.
While there is limited information on the specific market size of CSB, it is reasonable to assume that the market size is a fraction of the overall biodiesel market. This is due to the limited availability of cotton seeds for biodiesel production and the higher cost of producing CSB compared to other biofuels [38].
Conclusions & Outlook
Cottonseed oil has been considered a potential biodiesel production feedstock due to its high oil content and availability in large quantities. However, the future of cottonseed biodiesel (CSB) production remains uncertain due to several factors, including competition with food and animal feed industries, land-use concerns, and the emergence of more efficient and sustainable feedstocks. One of the significant challenges facing cottonseed biodiesel is competition with the food and animal feed industries. Cottonseed is an essential source of protein and has traditionally been used as animal feed. As such, the use of cottonseed for biodiesel production may result in higher prices for cottonseed meals, which could impact livestock and poultry industries. Moreover, cottonseed oil may also compete with other vegetable oils, such as soybean and canola, which are widely used for biodiesel production. Another concern is the potential impact of cotton farming on land use and the environment. Cotton farming is known to require large amounts of water and can contribute to soil erosion and pollution from agrochemicals. Using cottonseed for biodiesel production may exacerbate these issues, particularly if it increases cotton cultivation. Despite these challenges, the use of cottonseed oil for biodiesel production is still being pursued in some regions, particularly in countries such as India and Pakistan, where cotton is a major crop. Several studies have shown that cottonseed biodiesel can meet the required standards for fuel quality and has similar or better properties compared to other biodiesel feedstocks. Additionally, technological advances, such as genetically modified cotton plants, may lead to more efficient and sustainable cottonseed production for both food and fuel purposes.
References
[1] N. Balasubramanian and K. Steward, “Biodiesel: history of plant based oil usage and modern innovations,” Substantia, vol. 3, no. 2, pp. 57-71, 2019.
[2] S. P. Raj, P. R. Solomon, B. Thangaraj, S. P. Raj, P. R. Solomon, and B. Thangaraj, “History of biodiesel,” Biodiesel from Flowering Plants, pp. 7-14, 2022.
[3] D. Songstad, P. Lakshmanan, J. Chen, W. Gibbons, S. Hughes, and R. Nelson, “Historical perspective of biofuels: learning from the past to rediscover the future,” in Biofuels: Springer, 2011, pp. 1-7.
[4] L. Lin, Z. Cunshan, S. Vittayapadung, S. Xiangqian, and D. Mingdong, “Opportunities and challenges for biodiesel fuel,” Applied Energy, vol. 88, no. 4, pp. 1020-1031, 2011.
[5] J. P. Dorian, H. T. Franssen, and D. R. Simbeck, “Global challenges in energy,” Energy Policy, vol. 34, no. 15, pp. 1984-1991, 2006.
[6] B. R. Moser, “Biodiesel production, properties, and feedstocks,” In Vitro Cellular & Developmental Biology-Plant, vol. 45, no. 3, pp. 229-266, 2009.
[7] E. M. Shahid and Y. Jamal, “A review of biodiesel as vehicular fuel,” Renewable and sustainable energy reviews, vol. 12, no. 9, pp. 2484-2494, 2008.
[8] I. R. Fattah, H. Masjuki, A. Liaquat, R. Ramli, M. Kalam, and V. Riazuddin, “Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions,” Renewable and Sustainable Energy Reviews, vol. 18, pp. 552-567, 2013.
[9] F. Cavallucci, “Cottonseed oil: a possible feedstock alternative for renewable Diesel production,” 2020.
[10] M. N. Nabi, M. M. Rahman, and M. S. Akhter, “Biodiesel from cotton seed oil and its effect on engine performance and exhaust emissions,” Applied thermal engineering, vol. 29, no. 11-12, pp. 2265-2270, 2009.
[11] A. Srivastava and R. Prasad, “Triglycerides-based diesel fuels,” Renewable and sustainable energy reviews, vol. 4, no. 2, pp. 111-133, 2000.
[12] G. M. Mathew et al., “Recent advances in biodiesel production: Challenges and solutions,” Science of the Total Environment, vol. 794, p. 148751, 2021.
[13] M. Tabatabaei et al., “Reactor technologies for biodiesel production and processing: A review,” Progress in Energy and Combustion Science, vol. 74, pp. 239-303, 2019.
[14] J. Marchetti, V. Miguel, and A. Errazu, “Possible methods for biodiesel production,” Renewable and sustainable energy reviews, vol. 11, no. 6, pp. 1300-1311, 2007.
[15] T. Issariyakul, “Development of Biodiesel Production Processes from Various Vegetable Oils ” Ph.D., Division of Environmental Engineering University of Saskatchewan Saskatchewan, Canada 2011.
[16] M. N. B. Mohiddin et al., “Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review,” Journal of Industrial and Engineering Chemistry, vol. 98, pp. 60-81, 2021.
[17] M. Ramos, A. P. S. Dias, J. F. Puna, J. Gomes, and J. C. Bordado, “Biodiesel production processes and sustainable raw materials,” Energies, vol. 12, no. 23, p. 4408, 2019.
[18] M. Jayakumar et al., “Heterogeneous base catalysts: Synthesis and application for biodiesel production–A review,” Bioresource Technology, vol. 331, p. 125054, 2021.
[19] Y. Zhang, M. Dube, D. McLean, and M. Kates, “Biodiesel production from waste cooking oil: 1. Process design and technological assessment,” Bioresource technology, vol. 89, no. 1, pp. 1-16, 2003.
[20] B. Freedman, R. O. Butterfield, and E. H. Pryde, “Transesterification kinetics of soybean oil 1,” Journal of the American Oil Chemists’ Society, vol. 63, no. 10, pp. 1375-1380, 1986.
[21] H. Aksoy, I. Kahraman, F. Karaosmanoglu, and H. Civelekoglu, “Evaluation of Turkish sulphur olive oil as an alternative diesel fuel,” Journal of the American Oil Chemists’ Society, vol. 65, no. 6, pp. 936-938, 1988.
[22] G. Perez, “Analysis of enzymatic alcoholisis reaction with vegetables oils,” Master thesis, February, 2003.
[23] A. Demirbas, “Progress and recent trends in biodiesel fuels,” Energy conversion and management, vol. 50, no. 1, pp. 14-34, 2009.
[24] P. T. Vasudevan and M. Briggs, “Biodiesel production—current state of the art and challenges,” Journal of Industrial Microbiology & Biotechnology, vol. 35, no. 5, pp. 421-430, 2008.
[25] E. M. Shahid and Y. Jamal, “Production of biodiesel: a technical review,” Renewable and Sustainable Energy Reviews, vol. 15, no. 9, pp. 4732-4745, 2011.
[26] S. Chozhavendhan, M. V. P. Singh, B. Fransila, R. P. Kumar, and G. K. Devi, “A review on influencing parameters of biodiesel production and purification processes,” Current Research in Green and Sustainable Chemistry, vol. 1, pp. 1-6, 2020.
[27] M. Athar and S. Zaidi, “A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production,” Journal of Environmental Chemical Engineering, vol. 8, no. 6, p. 104523, 2020.
[28] M. Lapuerta, O. Armas, and J. Rodriguez-Fernandez, “Effect of biodiesel fuels on diesel engine emissions,” Progress in energy and combustion science, vol. 34, no. 2, pp. 198-223, 2008.
[29] R. Edwards, J.-F. Larivé, V. Mahieu, and P. Rouveirolles, “Well-to-wheels analysis of future automotive fuels and powertrains in the European context,” CONCAWE, European Council for Automotive R&D, JRC Joint Research Centre of the European Commission, Version 2c, 2007.
[30] A. K. Azad, M. Rasul, M. M. K. Khan, S. C. Sharma, and M. Hazrat, “Prospect of biofuels as an alternative transport fuel in Australia,” Renewable and Sustainable Energy Reviews, vol. 43, pp. 331-351, 2015.
[31] A. K. Agarwal, “Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines,” Progress in energy and combustion science, vol. 33, no. 3, pp. 233-271, 2007.
[32] D. O. Onukwuli, L. N. Emembolu, C. N. Ude, S. O. Aliozo, and M. C. Menkiti, “Optimization of biodiesel production from refined cotton seed oil and its characterization,” Egyptian Journal of Petroleum, vol. 26, no. 1, pp. 103-110, 2017.
[33] U. F. A. Service, “Oilseeds: World Markets and Trade,” 2023. [Online]. Available: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf
[34] E. I. Administration. “Cotton Seed Oil-Based Biodiesel Plant in West Texas Starts Production.” https://www.eia.gov/todayinenergy/detail.php?id=37832 (accessed 2023).
[35] A. Gashaw and A. Lakachew, “Production of biodiesel from non edible oil and its properties,” International Journal of Science, Environment and Technology, vol. 3, no. 4, pp. 1544-1562, 2014.
[36] MarketsandMarkets. “Biodiesel Market by Type, Application, and Region – Global Forecast to 2025.” https://www.marketsandmarkets.com/Market-Reports/biodiesel-market-235565133.html (accessed February, 2023).
[37] G. M. Insights. “Cottonseed Oil Market Size By Application, Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2021 – 2027.” https://www.gminsights.com/industry-analysis/cottonseed-oil-market (accessed February, 2023).
[38] M. Kumar et al., “Cottonseed Oil: Extraction, Characterization, Health Benefits, Safety Profile, and Application,” Food Analytical Methods, pp. 1-15, 2022.

 To all knowledge
To all knowledge