1 Introduction
Heat exchangers are used extensively in petroleum refining and chemical plants. Fouling is a common occurrence that results in a huge loss in energy recovery or a rise in pressure drop. Even if the distillation apparatus is perfectly built, it must be cleaned regularly. If fouling is detected, it can lead to a cascade of issues. Heat exchangers are frequently forced out of operation due to a significant rise in pressure drop rather than a reduction in heat transmission.
Efficient heat exchangers are essential for the successful running of oil refineries and petrochemical plants. However, fouling can build upon both the tube and shell sides of the heat exchangers over time. Heavy asphaltenes in the crude oil can build on the sides of the heat exchanger tubes in crude unit exchangers. Limestone and other elements in the cooling water system of the plant might get deposited on the heat exchanger tubes. Engineers try to size the heat exchanger tubes so that the speed through the tubes is as high as possible to increase the duration before fouling starts to obstruct flow. However, fouling is an inescapable fact when heat exchangers are used.
As the issue of fouling worsens, heat exchange is impeded, and efficiency suffers, leading to lower productivity and higher energy consumption. For example, when crude pre-heat exchangers clog, the temperature of the crude oil entering into the furnace drops, necessitating the use of more fuel to keep the furnace running. Fouling can result in greater tower pressures in tower overhead exchangers, reducing efficiency and fractionation effectiveness.
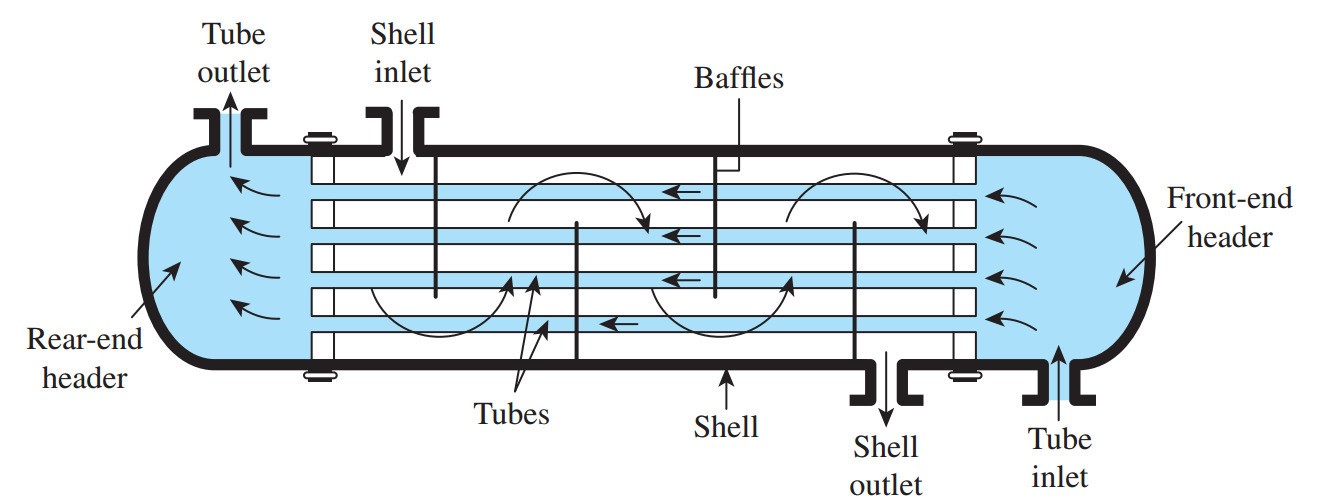
Figure 1 Shell and Tube Heat Exchanger
The maintenance cost needed to clean fouled heat exchange networks and the accompanying reduced productivity while the exchanger is out of service are two components of the high cost of fouling to oil refineries. This article will go through some specific fouling conditions in oil refineries that can be helped by chemical cleaning. Refineries administrators, experts, and chemical vendors need to work together to use the appropriate solvents and establish the best techniques for using them for cleaning.
2 Chemical Cleaning of Heat Exchangers
Conventional manual cleaning methods, custom-made chemical cleaning procedures, or a mix of both can be used to restore heat exchanger efficiency. High-pressure cleaning systems can take many days to mechanically clean large heat exchange networks. Traditional alkaline cleaning processes are still utilised in oil refineries and petrochemical industries to remove the organic part of residues such as grease and oil, accompanied by acid cleanup to disintegrate scaling and soften residues. However, the effects of such traditional cleaning operations are sometimes only modest, necessitating extra mechanical cleaning at significant expenditures [1].
When viscous and stubborn substances such as heavier hydrocarbons or additive compounds from upstream operations need to be removed, a mix of alkaline and acid cleaning is not the best option. In such cases, the high-performance solvent cleaning procedures may drastically minimise downtime while providing great results. Hydrocarbons, grease, asphaltenes, paraffin, natural oils, and other contaminants are removed from metal surfaces without expensive organic solvents. The chemical cleaning solutions may be water, circulated with a specialised apparatus. Deposition and thick layers are disintegrated into the cleaning solution, and heat exchangers are washed shorter using chemical cleaning.
Chemical cleaning is the method of cleaning heat exchangers to achieve less expensive and faster heat exchanger upkeep. Cleaning of a heat exchanger having fouling using a chemical solution to dissolve part or all of the elements of the solid foulant deposit is a wide definition. Cleaning is often accomplished by passing a solvent across the heat exchanger by circulation or once-through without disassembling the heat exchangers.
Reactive cleaning and decontamination are the two primary types of chemical cleaning procedures. Inorganic, non-carbon pollutants such as limestone, calcite, oxides, calcium phosphate, and sulphides are removed via reactive cleaning. When hydrocarbon pollutants are involved, decontamination chemistry is used. Chemical cleaning uses a mixture of three cornerstones: chemicals, circulation, and temperature in both reactive and decontamination cleaning. Chemical cleaning generally takes more preparation and expertise than hydro blasting since each of these foundations might affect effectiveness in eliminating deposits.
The most common application of reactive chemical cleaning is cooling water heat exchangers and boilers to increase fuel efficiency. Moderate to more powerful acids are cycled all through the network with inhibitors to eliminate pollutants while limiting impact on the base material, based on the kind of pollutant. It is important not to expose the tubes to the chemicals for longer durations since this might lead to metal decomposition. After cleaning, passivation is normally done using an alkali solution such as sodium carbonate, also known as soda ash, for carbon steel and an alkaline solution such as sodium carbonate, also known as soda ash, for stainless steel.
When hydrocarbon deposits in the fouling, decontamination is used to eliminate them. The decontamination employs various techniques, including pH adjustments and surfactant packages that target certain hydrocarbon types. As the chemicals are applied, circulation and temperature are regulated in various ways. Flow, filling and soaking, foaming, and even vapour-phase application can all be used to generate the proper flow. Direct steam injection or installing an extra heat exchanger are frequently used to regulate temperature.
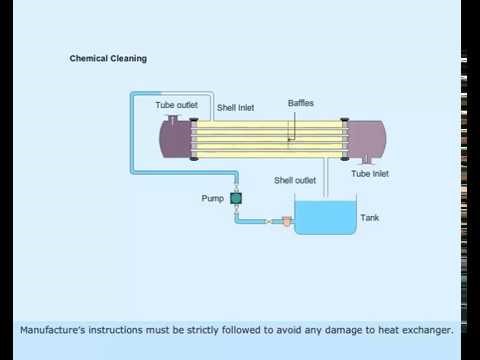
Figure 2 Chemical Cleaning of Heat Exchanger
3 The procedure of Chemical Cleaning
Dismantling of both ends and internal parts, separation of the tube bundle, carriage to a cleaning plant, cleaning, final assembly, and leakage testing are common procedures for large shell-and-tube heat exchangers. This operation can take anywhere from 4 to 15 days, based on various parameters such as the weight and size of the heat exchanger, the intensity of the fouling, and if the specialised gear is necessary to retrieve the tube bundle. The cost of physical work might be as high as 60k$ per exchanger in the worst scenario. Based on the time of the downtime and whether or not production is disrupted during the cleaning, a cost of up to or more than ten times the cost might be applied. As a result, there is a clear financial reason to design cleaning technologies that reduce or eliminate mechanical expenses and could be undertaken considerably less time [2].
Chemical cleaning is a method for achieving the goals of less expensive and faster heat exchanger repair and maintenance. Cleaning a heat exchanger with fouling using a chemical solvent to dissolve part or all of the elements of the solid contaminant deposition is the main process of chemical cleaning. Cleaning is often accomplished by passing a liquid chemical across the exchanger either through circulation or once-through without disassembling the system.
Chemical cleaning of heat exchange systems has only proven successful in a few circumstances. Even then, the advantages are only sustained for a short period compared to the other approach of dismantling and washing with a high-pressure water jet. The typical procedure is to pump petroleum streams such as kerosene or gas oil in the hopes of dissolving and washing away the contaminants and deposits, which are believed to be primarily organic. Chemical cleaning is not particularly efficient, as seen in the data present, which are extremely typical for such techniques. Chemical cleaning’s lack of effectiveness in various scenarios can be linked to two basic causes. First, the inefficiency of chemical cleaning as a solvent for both decomposed organic components in the contaminants and inorganic components, both of which might be present in the deposits. Secondly, due to the narrow couplings used for flow, it cannot circulate an adequately enough flow of solvent and get the solvent to cover all the zones.
Investigation and advancement in the key areas are necessary to reap the significant benefits that successful chemical cleaning may provide: (1) Assessment of which solvents can dissolve the various types of contaminant material can be circulated safely in oil refinery equipment, (2) Processes and apparatus to make sure sufficient distribution of the solvent to all zones of the fouled hardware, and (3) Knowledge of the fouling cycle so that regular maintenance can be conducted at the best possible repetitions taking into consideration expenditure, efficiency, and time needed for the procedure.
Fouling of oil products due to organic and inorganic precursor chemicals; fouling of sludge due to good catalyst particulate matter as well as corrosion problems; fouling of heavy hydrocarbon streams due to corrosion and coking, and fouling of mid-range hydrocarbons due to crystallisation are all examples of situations where significant cost savings can be realised. Oil companies, institutions, suppliers, and chemical businesses must work together to create or discover compounds that can be employed in certain fouling circumstances. The study goals should be establishing guidelines for such compounds to be utilised properly to get the most benefit at the lowest cost. Chemical cleaning of petrochemical plants’ heat exchange systems has the prospect to save much money. Still, it will take a lot of combined research and innovation among oil and gas companies and chemical businesses to get there.

Figure 3 Chemical Cleaning Process
4 Types of Chemical Cleaning in Heat Exchangers
4.1 Circulation Method
Feeding a heat exchange network with a chemical cleaning agent and pumping it around is how this approach works. In the chemical cleaning segment, this is the most popular procedure. To prevent rusting, it’s crucial to limit the liquid flow within a specified range. Moreover, the cleaning solution’s content and temperature must be checked during [3]. Like any other pickling process, this procedure should be used in three stages: degreasing, metal collecting, and passivation.
4.2 Soaking or Impregnation Method
When adequate circulation is not possible, the soaking method is frequently employed for inner surface cleaning of large capacity tanks and pipelines, for example, heat exchange networks, vessels, pipes, and boilers. Over time, the network is supplied with a chemical cleaning agent and emptied. This can be done multiple times if required until the apparatus is clean. Mineral acids can react with metal oxides with very little excitation. This method may be used for cleaning before and after operations [4].
4.3 Surge Method
The surge method is intended to be a low-pressure, fast way to get recognisable results in long pipes. This technique uses a very small amount of water to achieve higher speeds. Using an air receiver, rapid opening valve, and makeshift pipe to manage the outgoing water capsule, the surge method can remove grease adherent material and securely carry the removed objects out of the system. This technique provides quick pipe system cleaning without the requirement for cleaning chemicals to be disposed of. Because just a limited quantity of water is utilised in the procedure, removing and cleaning adhering material on the interior walls of piping is usually more successful. The purity of the water and air leaving the pipe and a visual check confirm a clean [5].
4.4 Vapor-Phase Method
This technique is meant to clean process plants in a single operation, making it quick and efficient—and hence cost-effective. Relatively large containers, boilers, heat exchangers, and interconnected pipes are process plants. Chemicals are put into the stream of steam in this improved steaming procedure. Despite its versatility, this technique is most commonly employed to remove hydrogen sulfide, phenol, combustible iron, combustion byproducts, and ammonia after operations. Degassing is a chemical cleaning procedure that removes potentially hazardous gaseous constituents from hydrocarbon processing facilities. Decontamination or solvent circulation is advised before degassing to reduce the number of contaminants and enhance the degassing process, for example, residue, heavy deposits, etc. Using a properly developed set of chemicals, any maintenance stop down during hot operations is reduced or eliminated. This technique eliminates both wastage and human contact with hazardous compounds [6].
5 Chemical Cleaning Fluids
To accomplish a comprehensive cleaning, chemical cleaning is frequently done in phases. The following factors and lab testing against specific fouling formations determine the appropriate type or mix of liquids or gases to employ. It is advised to use acids, alkaline solutions, oxidising agents, or solvents.
Cleaning acids come in a wide variety of strengths. HCl, nitric, sulphuric, and other acids are examples. Inhibitors are frequently added to prevent the acid from damaging the surface of heat exchangers. Organic contaminants such as oils and sludge are frequently removed using alkaline cleaning solutions like sodium carbonate and ammonia. Sodium bromate, potassium permanganate, and sodium nitrite, for example, establish a persistent oxide film on the cleaned surface that acts as a rust barrier. Passivation is the name for this particular cleaning technique. Solvents such as aromatic hydrocarbons, aliphatic hydrocarbons, and chlorinated hydrocarbons are utilised to remove certain deposits, although their usage is strictly restricted [7].
Corrosion inhibitors, detergents, inhibitors, antifoams, and solvents are all chemicals that may help in the cleaning process. Cleaning advice will be included in a comprehensive design process that considers your procedure. Your heat exchanger servicing professionals must be capable of guiding you on the appropriate chemical and additive mix to obtain the desired outcomes.
6 Advantages of Chemical Cleaning of Heat Exchangers
Some of the advantages offered by the chemical cleaning are:
- Cost-effective: In the chemical cleaning, the results are achieved without time-consuming and expensive unit disassembly and reassembly, lowering labour costs and minimising system downtime.
- Effectiveness: Compared to hydro-blasting, chemical cleaning significantly increases the system’s cleanliness. Depositions are usually removed completely, improving flow and heat transmission efficiency.
- Thoroughness: Thorough deposition cleaning reduces the seeding of new contaminants and increases the unit’s operating duration.
- Efficiency: Chemical cleaning is significantly quicker than other cleaning methods, particularly when dealing with complicated systems.
- Accessibility: Chemical cleaning has the advantage that it can be done in areas that are often unreachable.
- Online cleaning: chemical cleaning also provides online cleaning, which allows you to perform the cleaning process without shutting down the system.
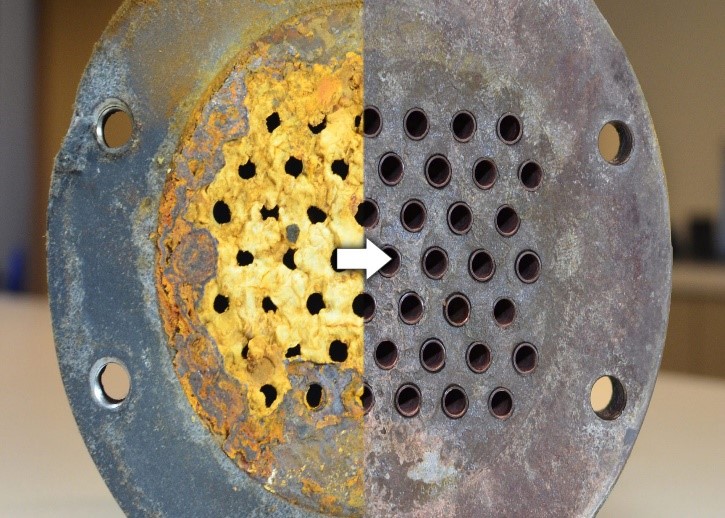
Figure 4 Chemical Cleaning Result
7 Disadvantages of Chemical Cleaning in Heat Exchangers
As compared to other cleaning techniques, chemical cleaning has a few disadvantages as well, which are listed below:
- Time: Chemical cleaning needs a proper planning before being carried out. Therefore, greater planning and scheduling needs demand more upfront time for chemical cleaning than other cleaning techniques.
- Waste disposal: Chemical cleaning produces a huge amount of waste which could be very hazardous. Therefore, this waste needs to be treated or disposed of properly, which requires more time and resources.
- Unexpected consequences: In the case of reactive cleaning, chemical cleaning eliminates all depositions, even those that may be aiding in tube strength and maintenance where tube walls have weakened.
8 References
[1] B. Wysocki et al., “The influence of selective laser melting (SLM) process parameters on in-vitro cell response,” International Journal of Molecular Sciences, vol. 19, no. 6, p. 1619, 2018.
[2] C. Boyd, F. Shadel, and J. Westsik, “FAST FLUX TEST FACILITY REFERENCE CONCEPT. Progress Report,” Battelle-Northwest, Richland, Wash. Pacific Northwest Lab.1967.
[3] R. Law, A. Harvey, and D. Reay, “Opportunities for low-grade heat recovery in the UK food processing industry,” Applied thermal engineering, vol. 53, no. 2, pp. 188-196, 2013.
[4] U. Midtgård, “Blood Vessels in the Hind Limb of the Mallard (Anas platyrhynchos): Anatomical Evidence for a Sphincteric Action of Shunt Vessels in Connection with the Arterio‐venous Heat Exchange System,” Acta Zoologica, vol. 61, no. 1, pp. 39-49, 1980.
[5] H. Gavel and H. Ellstrom, “Simulation of cavitation and pressure surge caused by rapid valve closure. An engineering approach,” in AIAA Modeling and Simulation Technologies Conference, p. 6676.
[6] D. Wilson, “Challenges in cleaning: recent developments and future prospects,” Heat Transfer Engineering, vol. 26, no. 1, pp. 51-59, 2005.
[7] C. Cacho, E. Turiel, A. Martin-Esteban, C. Pérez-Conde, and C. Cámara, “Clean-up of triazines in vegetable extracts by molecularly-imprinted solid-phase extraction using a propazine-imprinted polymer,” Analytical and Bioanalytical Chemistry, vol. 376, no. 4, pp. 491-496, 2003.

 To all knowledge
To all knowledge