1 Amine Gas Treatment: Introduction, Purpose, and Benefits
The greenhouse gases (GHS) present a danger to the well-being of the whole planet. Nowadays, all ecologically aware companies must remove them as efficiently as possible for the planet’s well-being. CO2 is the most abundant GHG, and it has gathered much attention in recent years due to pollution and climate change. Besides GHG, there are hazardous gases that are products of the industry. One of those gases is hydrogen sulfide gas. This gas is an easily flammable and highly dangerous gas that can leave severe consequences on human health. Hydrogen sulfide is a product of the decay of bioorganic matter. Therefore, it is commonly found in all types of crude oil. It is also a standard component in natural gas and liquid propane. Hydrogen sulfide can cause issues in pipelines and other metal parts of the equipment due to its highly corrosive effects. When H2s reach the atmosphere, they easily spread by diffusion in lower atmospheric layers due to their density, which is higher than the air’s. It irritates the eyes, nose, and throat at concentrations as low as 5 ppm. Concentrations around 30 ppm can cause paralysis of the sense of smell. This gas is lethal for humans when they are exposed to concentrations larger than 1000 ppm. The poisoning happens when the respiratory tract inhales gas. A concentration of H2S gas above 100 ppm is Immediately Dangerous to Life and Health (IDLH). Due to its toxicity, removing and constantly monitoring its concentration in plants is mandatory. It is essential to mention that hydrogen sulfide gas at higher temperatures burns during the oxidation process and generates toxic gases, such as sulfur dioxide.
It is essential to mention that even one of the fastest-growing renewable sources, geothermal energy, generates a tremendous amount of H2S gas, which has to be removed. Although their concentrations are far lower than those found in crude oils and natural gas, H2S gas is also a biofuel product. Although energy trends have changed in the last few years, the above gases will remain byproducts in this industry for a long time. Thus, highly efficient removal processes such as amine gas treatment are needed.
Amine gas treating, also known as amine scrubbing, gas sweetening, and acid gas removal, is a group of processes in which various aqueous solutions of various amines remove hydrogen sulfide (H2s) and carbon dioxide (CO2) from gases. H2S is a gaseous acid; therefore, the removal process got the name sweetening.
The amine gas treatment is used as a pretreatment of natural gas. Still, it is also used to remove the mentioned components in flue gas, which is the byproduct of the combustion processes in the industry. The process mechanism is quite the same in both situations. The same principles for the mass transfers are included in both cases, but equipment sizing, working parameters, and choice of the amines depend upon various conditions. The recently presented alternative for H2S removal, which uses filters with active carbon, showed many disadvantages. Firstly, this technology must include chemical impregnation and active carbon usage to be highly efficient. Secondly, it is more likely applicable just for treating flue gasses because high-pressure drops occur in the membrane and negatively affect the inlet stream of crude oil and natural gas. Lastly, equipment cost analysis shows that this technology requires high capital and operating costs. Conversely, amine gas treatment proved to be an economically beneficial process. Also, aqueous amines, primary inlet materials, can be easily purchased worldwide at affordable prices.
2 Input Materials and Chemical Mechanism of the Process
Alkanolamines are the aqueous amines commonly used as inlet material in the industry. The widely used alkanolamines are Monoethanolamine (MEA), Diethanolamine (DEA), Methyldiethanolamine (MDEA), Diisopropanolamine (DIPA), and Diglycolamine (DGA).
The reaction of aqueous ammonia with ethylene oxide industrially produces MEA and DEA. MEA and DEA are primary and secondary amines. They are highly reactive and can efficiently remove a high volume of CO2 and H2S due to a high reaction rate. The stoichiometry defines the constraint, and one mol of amine reacts with 0.5 mols of CO2 or H2S. However, stoichiometry limits the loading capacity to 0.5 mol CO2 per mole of amine. MEA and DEA also require a large amount of energy to strip the CO2 during regeneration, which can be up to 70% of total operating costs. They are also more corrosive and chemically unstable compared to other amines. Ammonia is one of the most abundantly produced inorganic compounds. It is a highly affordable material, and it has various purposes. Fourteen million tonnes of ammonia were produced globally in 2019, according to Statista analysis. Ammonia has to be converted in an aqueous state, and then it’s ready for amine production. In all production processes, ethylene oxide and ammonia react in a batch process that yields a crude mixture of ethanolamine, diethanolamine, and triethanolamine. The stoichiometry of the reactants controls the ratio of the products. All three products have different evaporation temperatures, so the distillation process easily separates them.
Every alkanolamine has its specific affinity towards CO2 and H2S. Alkanolamine mustn’t have an affinity toward methane and other light alkanes. Absorption as a mass transfer phenomenon can be represented by chemisorption and physisorption. For chemisorption, the solubility of the gas in the liquid significantly increases at low pressures, while at higher pressures, absorption affinity decreases. Alkanolamines are favourably used in industry due to their affinity for these gases. Most of the other solvents mainly physically absorb the gas, which can’t be as efficient as absorption where both transport phenomena occur.
Now, the question is why the weak base is used for absorption when a much stronger base can also be efficient. The answer is that alkanolamines are perfectly balanced compounds for absorption and regeneration. The alcohol group is an electron-withdrawing group, contributing to the compound’s polarity. Most of the alkanolamines possess pKa values that are only slightly lower than those of methylamines. This phenomenon is caused by an alcohol group, which creates hydrogen bonds to the amine’s proton of the same order. A lone electron pair from an oxygen atom forms an intramolecular hydrogen bond with a hydrogen atom from the amine group. So, the lone pair of nitrogen atoms is the only place available for the absorption of the proton. Research proved that intramolecular hydrogen bonds are crucial in determining the pKa values of alkanolamines and their temperature dependency. The pKa or alkalinity of the compound is not always a decisive factor for determining the kinetic constant, but it undoubtedly plays a significant role in kinetics.
Below are presented reactions of MDEA with CO2 and H2S:

Here, two processes co-occur. The lone pair of Nitrogen atoms gains a proton while CO2 and H2 are diluted and transferred into ionic forms. As we may notice, this is a reverse reaction; thus, ensuring proper conditions to favour the reaction in the desired direction is crucial. Firstly, a chosen amine’s concentration must be at least ten times higher than the concentration of the gases for successful absorption. Secondly, the chemical reaction of absorption is an exothermic process, which means that heat is released during the process. Both reactions present pseudo-first-order kinetics reaction, where rCO2 = k [CO2] and rH2S = k [H2S]. Where kinetic constant is expressed as:
![]()
Based on heat and equilibrium law, the reactants are abundant when the temperature is low; in this case, the direct reaction will dominate. When temperatures are higher, the reverse reaction will be the favourite, which is extremely important in regeneration.
3 Amine Gas Treatment: Process Flow Diagram, Equipment and Description of the Process
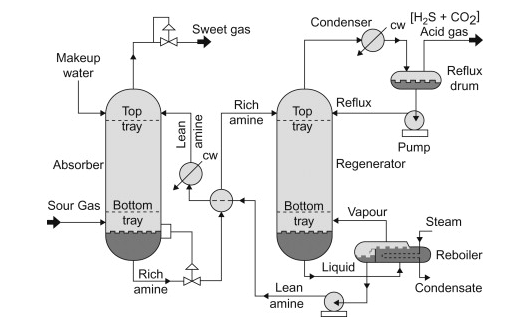
Common working conditions in the process units:
Absorber: 35 to 50°C and 5 to 205 atm of absolute pressure
Regenerator: 100 to 126°C and 1.4 to 1.7 atm of absolute pressure at the tower bottom
It is common engineering praxis to maintain the temperature difference between lean amine and sour gas at least 5°C. If the temperature difference is closer, the condensation of hydrocarbons could occur, which is not acceptable in this process. In this process’s design, most sources recommended a steam ratio, defined as the mass flow of steam per volume of amine circulation. Most of these sources propose 0.12 kg/l as an optimal value for the steam ratio.

Residual H2S for an MDEA System at feed gas H2S/CO2 ratios of 0.25, 0.5, 1, 1.5, 2, 4, and 10
The absorption process occurs while fluids are flowing in a counterflow direction. The aqueous stream flows downward, while the gaseous stream flows upward. Based on the proposed temperature and pressure suggestion, the optimal number of trays in most cases is 22.
The outlet stream at the top of the absorber presents the stream of natural gas without the presence of CO2 and H2S.
From the physisorption perception of view, the driving force in the absorption process is the partial pressure of the particular gas. Thus, absorption would be impossible under low pressure without the proper reboiler’s duty. The interesting fact derived from recent process optimization is that higher temperatures than 104 degrees Celsius do not add any benefits in terms of energy efficiency. At first sight, this fact can sound illogical for an engineer, but it is indeed apparent when the overall process is integrated in terms of material and energy balance. The graphic below shows that a minimum heat demand is reached at 104 degrees Celsius.

As we may notice from the process flow diagram, a recirculation stream of rich amine is at the bottom of the absorber. This stream is not a typical recirculation stream, where one part of the stream continuously recirculates to ensure the process efficiency. Every alkanolamine has its saturation level of CO2/H2S at a specific temperature. Thus, the control system of the recirculation stream should be designed in the following way. A control valve that controls the inlet stream of the regenerator should be normally closed. On the other side, the rich amine should recirculate until it reaches the saturation level. When that level is achieved, the actuator opens the outlet valve, reaches the amine and travels to the regenerator. It is also essential to continually control the flow of the aqueous inlet stream. With tight constraints regarding the desired inlet flow, flooding will be avoided. In this way, the operation costs of the process are minimized due to proper energy usage.
Another example of efficient energy usage in this process is the heat exchanger, where the lean amine transfers heat to the rich amine. With this design, it is impossible to achieve that energy from lean to rich amine is perfectly transferred. Hence, the reach amine has to be even more cooled by chilled water, which is a viable solution due to the abundance of water used as raw material.
The last step of the amine gas treatment presents desorption, widely known as gas regeneration. As we said earlier, the alkanolamines are perfect compounds in this case because proper conditions can achieve absorption and desorption. Therefore, the expansion valve reduces the pressure of the reach amine. When the pressure of the liquid is reduced, the partial pressures of the C02 and H2S rapidly approach their saturation pressure. The rise of the temperature to a proper value enables the evaporation of gases in the regenerator. The liquid-vapour equilibrium is formed at every trace. Gases as lighter substances travel upwards, and lean amine in the form of liquid travels downwards.
The liquid-vapour equilibrium is also achieved in the reflux drum. The gas from the top of the regenerator is firstly condensed and then enters the reflux drum. This step is involved to ensure that no or minimal amount of alcohol amine exits from the system. That leads us to the conclusion that amine continuously circulates in both process units. It changes its composure but is not part of the outlet stream. Pump pushing reflux back to the regenerator doesn’t require a lot of energy due to minor pressure demands in the regenerator.
The reboiler heat demands were explained according to the energy needs of the rich amine stream. It is crucial to notice that the reboiler is also a flash drum. As we said earlier, the rich amine stream is saturated with gases, and it is most likely that some percentage of gases would appear in the flash drum. To avoid that, engineers must design the regenerator with sufficient trays. One of the common issues that come with regenerator design is oversizing. Oversizing of the equipment leads to overwhelming investment costs. On the other side, keeping residual acid gases at the bottom of the regenerator under the permitted level is essential. Otherwise, acidic gases travel at the top of the absorption column, where most will evaporate. That undesired scenario produces insufficiently clean natural gas, and the H2S concentration in the sweet gas will be too high to meet pipeline specifications.
4 Amine Gas Treatment: Discussion About Design and Conclusion
Besides recommended parameters and values, an engineer must know many factors when designing an amine gas treatment facility. The composure of natural gas varies from place to place, and it has to be examined. Based on the natural gas composure and availability of amines, an engineer must choose the proper alkanolamine that will satisfy all criteria. Typical concentrations are optimal for this process depending on which particular amine is chosen.
The cooling and heating utility is water, a highly affordable material anywhere. The last concern about the design is the proper fuel for heating the water. The best solution for reaching an even more efficient process is using natural gas, the exploitation’s target compound. The combustion of natural gas releases heat that could generate the needed steam. That would be the proper solution for refineries. On the other side, thermal and geothermal plants should use the remaining heat of the flue gases to heat the water and transform it into steam. Application of the cogeneration principle, in this case, would be highly beneficial for a significant reduction of operating costs.
The overall efficiency of the process is extremely high. The acidic gases are removed, which is the main and only purpose. There is still a lot of space for further energy conservation and process optimization improvements, with a minor increase in investment costs. Thus, amine gas treatment has a bright future and firmly holds the first place in flue gas treatment.
Read more about EPCM’s Experience.

 To all knowledge
To all knowledge