1 Air Separation of Cryogenic Gases: Current Technologies
An Air Separation Unit (ASU), is a type of technology that separates air into its main constituents.
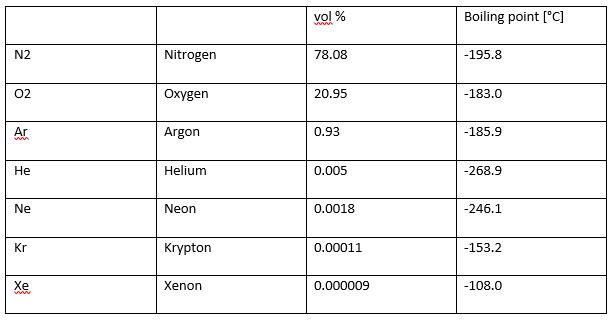
The most abundant components are Nitrogen, followed by oxygen and then argon, as well as other inert gases in small amounts. The compositions are summarised in table 1 below.
There are various methods or technologies that can be used to separate air into its components, the oldest and most popular being cryogenic or fractional distillation. Other methods include pressure swing adsorption (PSA) as well as membrane technology, but hybrids of these technologies also exist and new and improved methods are being researched. These three methods are explained in the sections that follow.
Table 1: Composition of dry air (Adapted from Linde Engineering, © 2017)
Oxygen, which makes up 21% of air by volume, is the most widely used air product with many industrial applications. The production of oxygen from air separation is a large and growing industry, producing almost 100 million tons of oxygen annually (Hashim, 2011). The oxygen market is growing, as all future clean energy technologies will need to be fed oxygen. The air separation methods mentioned above are used to separate oxygen from the air, but are also used to obtain other air products, with various by-product recoveries. These methods also vary with the scale with which they can be used. The effectiveness of these technologies is summarised in table 2.
Table 2: The comparisons of process alternatives for oxygen production from air separation (Adapted from Hashim, 2011).

In order for air to be separated into its different components, the air first needs to be liquefied (liquefaction of air). A gas becomes a liquid when its pressure and temperature are lower than that of its critical conditions, or critical point. The critical temperature of the air is -140.7°C (132.5 K) and the critical pressure is 37.7 bar. This means that air has to be cooled below this temperature in order to become a liquid and then be separated. This critical temperature varies depending on the pressure of the gas, in that a higher temperature is required for lower pressure. This relationship is proportional, but not linear (Linde Engineering, © 2017).
1.1 Cryogenic Air Separation
In 1895, Carl von Linde successfully liquefied air, attempting to obtain liquid carbon dioxide to be used in the brewing industry (Flavell-While, 2010). It was only later that separation methods were developed. This liquefaction process took three days. Separation methods have come a long way since then. Below is an early diagram of the liquefaction process.

Figure 1: Von Linde’s original drawing of his air liquefaction process (Flavell-While, 2010)
Cryogenic air separation is the most common and standard technology used for the separation of air into its constituents. It results in a high purity product and is the most developed method currently (Hashim, 2011). This is a highly energy-intensive process due to the low temperatures that need to be achieved, as can be seen from Table 1 above.
The air is first filtered, compressed and then chilled to -185°C. This liquefies the air, and this liquid stream is sent to a distillation column in order to be separated. This uses the natural temperature gradient within the column to allow nitrogen to leave the top of the column as gas and oxygen as a liquid at the bottom. Argon can also be separated by taking a stream somewhere in the middle of the column at the point where the argon concentration is the highest and feeding this into another column where nearly pure argon is separated from the other gases.
Some improvements to this process included using packed towers instead of traditional trayed towers, as well as introducing heat integration, and more efficient compressors in order to reduce the energy consumption. Argon separation has also been improved. Conventionally, catalytic converters using hydrogen were needed, but modern processes only require structured packings. This method is reaching its maturity level, and industries are looking at the other air separation methods, which are continually being developed, as there is potential for high purity and lower energy consumption (Hashim, 2011).
Below are more detailed steps for a typical cryogenic air separation process (Linde Engineering, © 2017). This is the process for the production of gaseous pure oxygen and nitrogen, as well as liquid oxygen, liquid nitrogen and liquid argon, with internal compression. A diagram for this process can be seen in figure 2.
- Air compression: Ambient air is compressed, using a multi-stage turbo compressor with intercoolers. Dust particles are removed using a mechanical air filter as air enters the compressor.
- Air cooling and & purification: The process air is cooled with cooling water, using a direct contact cooler, and soluble air particles are also removed. Cooling water is chilled in an evaporation cooler, utilising dry nitrogen waste gas from the separation process. Sieve absorbers are used to remove CO2, water vapour and hydrocarbons from the process air.
- Low-temperature heat exchange: Counter-current heat exchangers cool the process air to a temperature close to the liquefaction temperature, also utilising the nitrogen waste gas from the separation process.
- Cold production & internal product compression: A side-stream of process air is compressed using an air booster compressor. Cold production and expansion of this stream are achieved in an expansion turbine. A side-stream of this is further expanded and liquefied in a liquid separator. High-pressure heat exchangers are used to evaporating the pumped oxygen and nitrogen product and warm to ambient temperature.
- Cryogenic rectification of air: The cooled and liquefied air is pre-separated in a pressure column into an oxygen-enriched liquid at the bottom of the column, and pure nitrogen gas at the top. A condenser at the top is used for liquefaction of the pure nitrogen gas, and a reboiler at the bottom for boiling the oxygen, in order for greater product purity.
- Cryogenic rectification of argon: Further columns are needed to separate Argon. An argon-enriched side stream from the low-pressure column is separated from oxygen within the crude argon column. The liquid oxygen is pumped back from the argon column into the low-pressure column. Nitrogen is removed in a similar way in the pure argon column.

Figure 2: Typical cryogenic air separation process (Linde Engineering, © 2017)
1.2 Pressure Swing Adsorption
The basis of pressure swing adsorption (PSA) is that when gases are put under high pressure, the gases are attracted to solid surfaces and hence are adsorbed. This is a proportional relationship, in that the higher the pressure, the more gas is adsorbed. The adsorbent bed is known as a zeolite.
Filtered air enters the PSA generator which contains zeolites that absorb nitrogen and/or argon. This is done at high pressures, and these high pressures cause the gases to be attracted to the solid surface of the zeolites. Purified oxygen can then be separated. Once the oxygen has been collected, the pressure then swings to low pressure in order to desorb the nitrogen, which can also be collected. As soon as the pressure is reduced again, the gas is desorbed (released). This additionally frees up the adsorbent, ready for the next cycle.
Different gases can be adsorbed by using different solid particles, based on what the gases are more easily attracted to, so this method typically has relatively high product purity, although by-product purity is still being improved. If the zeolite attracts nitrogen, then some or all of nitrogen will be adsorbed when a stream of air is pressurised and can be released after it is depressurised again. The exiting stream will, therefore, be oxygen-rich, compared to the entering stream, and this oxygen will be collected. The zeolite can thereafter be reused for another batch. If two adsorbent vessels are used, this process becomes almost continuous (Ruthven, 1990). This is illustrated in figure 3 below.
This is the most suitable method for the production of oxygen for small to medium scale plants (20-100 tons/day). Large-scale plants typically use cryogenic separation (more than 100-300 tons/day). This is also an older technology; hence it has been perfected over the years in terms of adsorbents used, and energy consumption. This is the favoured alternative to traditional cryogenic distillation, as it has a significantly lower energy demand and a high product purity. There is a range of variations of PSA, including vacuum swing adsorption, temperature swing adsorption, vacuum-pressure swing adsorption, and many more. These systems rely on the zeolites to trap nitrogen and hence produce oxygen with a purity of 90% to 95%. Different adsorbents are being investigated continuously, in order to improve this process even more. The performance of this process is highly dependent on the pressure, which can be more energy-intensive, as greater energy is required for greater pressure and hence greater performance (Hashim, 2011).
The original pressure swing air separation process uses a zeolite adsorbent that is selective to nitrogen and hence produces an oxygen-rich exit stream. This method is predominately in small scale units, but many modifications have been made to adapt this method for large scale units, to reduce energy demand. Nitrogen can theoretically be recovered from desorption of the zeolite, but this is not of very high purity, so it is more desirable to use a zeolite that is selective to oxygen. This, however, is still being perfected (Ruthven, 1990).
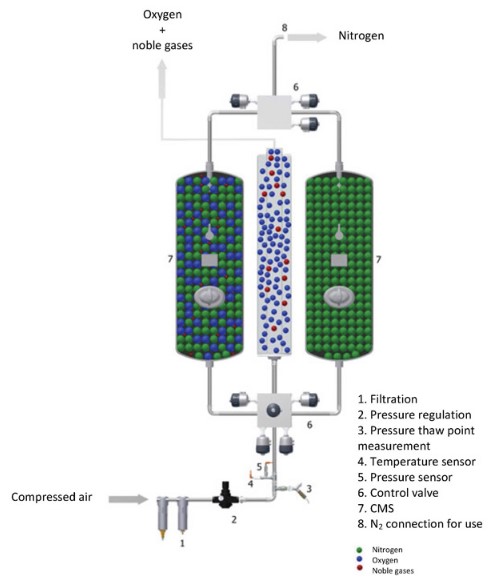
Figure 3: Pressure Swing Adsorption Technology (Inmatec, © 2017)
1.3 Air Separation of Cryogenic Gases: Membrane Separation Technologies

Figure 4: Schematic representation of the oxygen transport in dense MIEC ceramic membrane (Hashim, 2011)
The third type of air separation is via membrane separation technologies. This is a modern technology that is not yet well established but is potentially very promising. This is the most recent technology and new developments are constantly emerging from this, such as new types of membranes and hybrid systems involving both cryogenic distillation and pressure swing systems. Currently, dense ceramic membranes are used. This technique can separate oxygen from the air, usually at high temperatures of 800-900°C. This method also has high by-product purity recovery, so can be used to potentially separate out any component of air (Hashim, 2011).
Traditionally, ceramic membranes with mixed ionic electronic conducting (MIEC) characteristics have been looked at because this has been known to potentially produce high purity oxygen. This type of membrane actually does not need electrodes or an external circuit to run. In figure 4 above, it is illustrated that the oxygen partial pressure gradient actually creates an internal short circuit through the electronic conductivity (Hashim, 2011).
This method separates oxygen from air by taking advantage of the oxygen partial pressure gradient. There is a high oxygen partial pressure side of the membrane on the feed side and a low one on the sweep side. The oxygen naturally permeates from the high-pressure side to the low-pressure side. The flux of electrons through the membrane keeps the overall charge neutral. As the gas stream flows along the membrane, it is depleted of oxygen through the permeation through the membrane via the pressure gradient, and the sweep stream becomes oxygen-enriched. This sweep stream flows counter currently to the air feed. This oxygen is then collected (Hashim, 2011).
This technology is still very new and is being researched. There is a potential for a very high-quality product, and separation is possible at high temperatures (liquefaction is not necessary). Although this is not yet widely used, it is predicted that this will be the dominating method in the industry in the future (Hashim, 2011).
2 References and Bibliography
References:
- Hashim, S.S., Mohamed, A.R. and Bhatia, S. (2011). Oxygen separation from air using ceramic-based membrane technology for sustainable fuel production and power generation. Renewable and Sustainable Energy Reviews, [online] 15(2), pp.1285-1286. Available at: https://ac.els-cdn.com/S1364032110003424/1-s2.0-S1364032110003424-main.pdf?_tid=7fa3c5d8-dbe2-11e7-8447-00000aacb35d&acdnat=1512715331_569b9f52af38588da131d38badeb3d70 [Accessed 15 Dec. 2017].
- Linde Engineering (© 2017). History and technological progress: Cryogenic air separation. pp.1-20. Available at: https://www.linde-engineering.com/internet.global.lindeengineering.global/en/images/AS.B1EN%201113%20-%20%26AA_History_.layout19_4353.pdf [Accessed 15 Dec. 2017].
- Flavell-While, C. (2010). Carl von Linde and William Hampson – Cool inventions. [online] The Chemical Engineer. [Accessed 15 Dec. 2017].
- M.Ruthven and S.Farooq (1990). Air separation by pressure swing adsorption. Gas Separation & Purification, [online] 4(3), pp.141-148.
- (© 2017). THE INMATEC PSA TECHNOLOGY. [online] [Accessed 15 Dec. 2017].
Bibliography:
- This is how air separation works. (© 2017). Messer, pp.1-2. Available at: http://www.messergroup.cn/Products/down/LZAEnglisch.pdf [Accessed 15 Dec. 2017].
- Shepherd, M. (1941). ANALYTICAL SEPARATION AND PURIFICATION OF GASES BY FRACTIONAL DISTILLATION AND RECTIFICATION AT LOW TEMPERATURES. Journal of Research of the National Bureau of Standards, [online] 26, pp.227-244. Available at: http://nvlpubs.nist.gov/nistpubs/jres/26/jresv26n3p227_A1b.pdf [Accessed 15 Dec. 2017].
- Sally Berge

 To all knowledge
To all knowledge