Continuous Carbon Capture Process: In recent years, greenhouse gases have been identified as the major culprits of climate change, and carbon dioxide constitutes as much as 70% of the greenhouse gases emitted (U.S. Department of Energy, 2007). As a result, carbon taxes are increasingly being implemented worldwide to curb carbon dioxide emissions. Technologies that can capture carbon dioxide effectively and with a low parasitic load on the system will enable any operator emitting carbon dioxide to generate financial savings while contributing to the preservation of our environment.
Many processes exist on an industrial scale that capture carbon dioxide, but all of these processes are extremely energy-intensive. The main reason these processes are so energy-intensive is due to the batch or cyclic nature of the processes. Changing the temperature or pressure in the carbon dioxide capture system requires large amounts of energy to drive these processes. A continuous carbon dioxide capture process will, per definition, be more energy efficient.
This article documents the current hypothesis for developing and testing a continuous carbon dioxide capture system using Stirling Coolers as the driving force.
1. Continuous Carbon Capture Process: Stirling Cycle Refrigeration
A Stirling cycle cooler is a member of a family of closed-cycle regenerative thermal machines, including heat pumps and refrigerators, known collectively as Stirling cycle machines. In any refrigeration cycle, including the reversed Stirling cycle, work input is required according to the second law of thermodynamics. This is achieved by shuttling the gas in the system backwards and forwards between the hot end and cold end spaces so that the system’s temperature during compression is, on average, higher than during expansion. As a result, the work done on the gas during compression is greater than the work done by the gas during expansion, as illustrated in Figure 1. Accordingly, the hot and cold end gas spaces are also referred to as the compression and expansion spaces, respectively. Furthermore, for operation as a refrigerator, heat must be rejected via a heat exchanger at the hot end, and heat must be absorbed from the space to be cooled via a heat exchanger at the cold end.
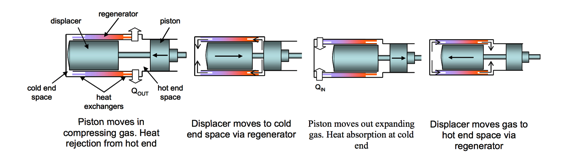
Figure 1. Piston and Sisplacer Movements During a Stirling Refrigeration Cycle.
Stirling engines have the advantage of being able to effectively convert heat energy as a driving force into mechanical work. Stirling coolers reverse the cycle, through supplying a mechanical driving force, heat energy is generated and removed by a heat exchanger, thus the cold end of the cylinder incrementally reduces its temperature to reach very low temperatures for relatively little work done. The figure below (source: Ray Radebaugh (NIST) 1999) illustrates Stirling cryocooler efficiency at 80K compared to traditional refrigerant, which is driven by cryogenic cooling systems.

Figure 2. Cryocooler Performance Comparison
2. The Conceptualisation of Continuous Carbon Capture Process
Cryogenic Carbon Capture processes have proven to work well, regularly recovering over 95% of the CO2 content in the gas stream. Due to the relatively high sublimation temperature of CO2, this is basically a single step. Typically, the condensation or sublimation temperatures for effluent or hydrocarbon gasses are much lower, enabling a very successful separation of the CO2.
As seen in the phase diagram below, for gas pressures below 5.1 1 atm, no liquid phase for CO2 exists, and thus, it sublimates directly from gas to solid state. This enables cryogenic processes to directly freeze out the CO2 from a gas stream below 5.11 atm.

Figure 3. Carbon Dioxide Phase Diagram.
Freezing out the CO2 directly from a gas phase requires a surface for the crystals to propagate on, which is the limiting factor in the existing cryogenic carbon capture processes. This limitation gives them batch characteristics and requires energy state changes for operation. To make a continuous cryogenic carbon capture process more energy-efficient and feasible for industrial use, an infinite surface area for CO2(s) propagation would be required. Traditional heat exchanger design clearly doesn’t allow an infinite surface area, which would, in any case, require an infinite amount of energy to drive the sublimation of the CO2.
Since our aim is a continuous process that doesn’t require cyclic changes in temperature or pressure to remove the CO2(s) from the gas stream, we clearly need to think creatively about heat exchanger designs.
2.1 Continuous Carbon Capture Process: Intended Process
To ensure the heat exchanger conforms to the process requirements, we must define the processes for removing the CO2. Our organisation’s current need is to enrich a bio-gas feed stream from an anaerobic digester (removal of CO2 and water vapour). The typical composition of the bio-gas contains more than 38% CO2 and varying amounts of H2O vapour, with the main constituent being methane (CH4), less than 4% nitrogen (N2) and very low concentrations of hydrogen sulfide (H2S). Since the primary objective is the removal of CO2 and the removal of water vapour is well established, we will only focus on CO2 removal as a process constraint. This process will typically be included after the H2O vapour has been expelled from the system.
2.2 Heat Exchanger Conceptualization
Since it has already been established that a conventional heat exchanger design will not meet our requirements, we must utilize a different approach for the required heat exchange. The author’s approach was to conceptualize a mass transfer process rather than focusing solely on energy (heat) transfer. This process also exchanges energy and provides an opportunity to separate different components based on their phase change properties.
The first process that comes to mind is a traditional scrubber column, which contacts liquid and gas streams to separate components from one stream, with the components being absorbed by the other. This process also facilitates energy exchange between the streams. If the selected liquid stream does not dissolve any components of the gas stream, nor is dissolved into the gas stream, only energy transfer will occur, functioning as a “Direct Contact Heat Exchanger.”
The liquids stream, cooled down sufficiently low, and if sufficiently dispersed (while ensuring sufficient mass to ensure heat exchange from the liquid to the gas can still occur), will increase the surface volume of the “heat exchanger” substantially, supplying a very large area for the propagation of the CO2 crystals. Consequently, CO2 crystals form on the surface of the heat exchanger liquid droplets. If the liquid stream and solid CO2 are separated, the liquid can be cooled again and recycled to the scrubber column, creating an infinite surface area for CO2 crystal propagation and enabling true continuous cryogenic carbon capture.
2.3 Heat Exchanger Component Conceptualization
Since the preliminary idea for a Direct Contact Heat Exchanger (DCHE) with a theoretical infinite surface area has been established, we must conceptualize the supporting equipment to enable its successful operation.
As discussed in the above section, the preliminary equipment will be based around an absorption or scrubber column. Typically, these columns come in various arrangements depending on the intended separation of components from the different feedstocks. For the DCHE arrangement, a counter-current scrubbing column is recommended to optimize the temperature difference (DT) between the gas stream and the heat exchange liquid (similar to the concentration difference in a mass transfer process).
Since the propagation of the CO2 crystals occurs on any possible surface and can consequently create system blockages, the internal design of the scrubbing column should not include any trays and should only be composed of a single spray head (possibly multiple nozzles to maximize the liquid dispersion).

Figure 4. Proposed Scrubber Column (single head layout).
The next step would be to conceptualize the heat-exchange liquid. This approach is very straightforward. As mentioned in the preliminary constraints, the heat exchange fluid should not allow any mass transfer from the gas stream, including the CO2, to it, or allow any of its mass to be transferred to the gas stream or the CO2 stream. Further, since we will be operating the column below the sublimation temperature of the CO2, the heat exchange liquid should have a lower freezing point while remaining sufficiently viscous at the operating temperature to allow for easy pumping and dispersion.
A detailed investigation is needed to identify a suitable heat exchange liquid. Hydrocarbon ring compounds containing fluorine (such as polytetrafluoroethylene) or chlorine atoms immediately come to mind.
It is reasonable to expect that the CO2 crystals forming on the surface of the cold heat exchange liquid will create a slurry when collected. The next step is to conceptualize the separation process for the liquids and solids.
Yet again, it is easier to turn to existing technology and possibly change it slightly to fit our process requirements. A 2-phase decanter centrifuge employs a rotational moment to separate components of different densities, making it ideal for slurry separation.
In typical horizontal decanter centrifuges, the rotating assembly is mounted horizontally with bearings on each end of a rigid frame, which provides a good sealing surface. The typical arrangement must be adapted to separate the CO2 crystals from the heat exchange liquid. Instead of the typical horizontal arrangement with the feed being fed in the middle of the centrifuge, the proposal is to orientate the decanter centrifuge at 45°, with the scroll and housing submerged under the liquid level, while employing a preliminary porous scroll housing to allow the heat exchange liquid to drain. The scroll discharge screw then forces the solid CO2 crystals to one end of the bowl to be discharged as solid CO2 ice. This arrangement will allow the centrifuge to function as a scroll feeder with a higher-than-normal rotational force and possibly compress all the liquid out of the solid CO2 by changing the scroll vane geometry.
As discussed in Section 2 above, the heat exchanger liquid’s cooling source will be a Stirling engine.
3. Continuous Carbon Capture Process: Conceptual proposal for further development
The conceptual idea for the “direct contact heat exchanger” and its supporting equipment has been established and needs to be defined for feasibility development. This design process set point is required for evaluating the preliminary concept and will assist in developing it into a feasible proposal. From this feasible proposal, a working pilot facility can be constructed to prove the concept and develop it into an acceptable process for industrial applications where needed.
The process layout to be tested for feasibility is illustrated below:

Figure 5: Conceptual Layout of Envisioned Continuous Carbon Capture Process.
The above layout, along with the descriptions in the previous sections, will serve as the conceptualization set point from which further feasibility studies will proceed. All components will be theoretically assessed for their ability to deliver on their intended requirements individually, and then the process will be assessed as a unit.
4. Next Steps
EPCM Consultants have proposed the above concept to the University of Pretoria’s engineering department in South Africa to assist with its feasibility development. A team selected by the university will carry out further development to create a feasible proposal and, ultimately, a working prototype. All progress will be included in future revisions of this article.

 To all knowledge
To all knowledge