The invention of synthetic plastic or polymer in 1907 and its fast commercialization around 1950s resulted in technical improvements affecting many aspects of human life [1]. Plastics, for example, have significantly contributed to computers, mobile phones, cheap automotive parts, flooring and roofing materials, and the majority of modern lifesaving medicine discoveries, among many other applications. Plastics are also frequently used in electronic and electrical areas and have good insulating and dielectric properties [2].
We have developed a rising addiction to this cutting-edge material, the majority of which are single-use items, as a result of the growing number of waste and associated complications.
Plastics have become more widely produced and used in recent decades. Globally, roughly 8,300 Mt of virgin plastics were produced between 1950 and 2015, leading to approximately 6,300 Mt of waste, of which 9% was recycled, 12% was burned, and 79% accumulated in dumping sites [3].
As a result, the amount of post-consumer plastic waste in the environment is rapidly rising [4]. Over 300 million tons of plastic are produced annually, continuously rising with time [5]. In many countries, waste produced by careless disposal of old plastics accounts for a considerable fraction of the total waste stream. The fact that aquatic species ingest marine plastic trash and break it down into microplastics and nano-plastics is even more concerning. According to research, this poses a major threat to the number and mortality of zooplankton, a vital energy source for the marine ecosystem.
Plastic wastes pose a hazard to the world economy, as well as to humans, animals, and the ecosystem. This is particularly true in developing countries, where there are few modern recycling infrastructures and weak legislation on plastics manufacturing, use, and disposal [6].
What is Plastic Waste Pyrolysis?
Plastic pyrolysis is a chemical reaction that includes heating larger molecules to convert them into smaller ones.
The Greek words “Pyro” and “Lysis” imply “fire” and “break down,” respectively. So, pyrolysis translates to “heat or fire breakdown.” Other names for pyrolysis include thermal cracking, the breakdown of polymers, and thermolysis. In the pyrolysis process with the assistance of heat and without an oxygen supply, plastic is converted into liquid oil.
Plastic waste can be converted into energy in solid, liquid, and gaseous fuels by the comprehensive close-loop method under pyrolysis [7].
Contrary to mechanical recycling, pyrolysis appears to be an appropriate method for recovering materials and energy from waste plastic without selecting a particular batch of thermoset or thermoplastic, and sorting it into different plastic types offers an effective way to reduce toxic compounds.
How do Plastic Additives Affect Humans and Animals?
Apart from the base plastic complications in safe disposals, plastic additives also pose significant challenges. The following are examples of additives that may include in plastics, depending on their type, intended use, and service environment:
- Plasticizers
- Antioxidants
- Fire retardants
- Light stabilizers
- Pigments
- Lubricants
- Thermal stabilizers
- Antistatic agents
Endocrine-disrupting chemicals (EDCs) are substances that imitate, inhibit, or otherwise interfere with hormonal activity in the endocrine system of the body. Research has revealed that many of these compounds enter the systems of humans and animals [8]. Additionally, many of them have been classified as persistent organic pollutants (POPs), commonly called “forever chemicals.” They are organic substances that are unable to be destroyed by biological, chemical, or photolytic processes in the environment. The majority of flame retardants include hexachlorobenzene, perfluorooctanoic acid (PFOA), polychlorinated biphenyls (PCBs), hexabromobiphenyls (HBB), polybrominated diphenyl ethers (PBDs), short-chain chlorinated paraffin (SCCPs), hexabromocyclododecane (HBCD), etc.
Although plastics have proven to have outstanding characteristics and uses for modern technological advancements, improper use, improper waste disposal, and lack of regulation on additives usage in plastics may result in the accidental discharge of toxic chemicals, which increases by their open combustion [9]. In order to avoid contamination from used and discarded plastics, authorities throughout the globe have switched to a non-scientific strategy, for example, waste plastic buyback, and a partial or complete ban on particular kinds of plastic goods [10]. Nonetheless, these policies have not had an important beneficial effect due to negative economic, legislative, political, technological, and operational constraints.
Traditional Method for Handling Plastic Waste
Landfilling
Landfilling is a common traditional waste disposal method in many countries, yet there is a shortage of landfill areas as a result of urbanization and population growth. Landfilling plastic garbage is currently the least preferred waste management method due to rising health and environmental concerns. A significant public health concern is the types, amounts, and potential for leakage of harmful substances into groundwater at landfill sites.
Open Burning
The open combustion of plastics in the form of municipal solid waste is a traditional approach that is still common in most communities worldwide, particularly in developing and poor countries. One of the main causes of air pollution is the combustion of plastic garbage in open spaces. Around 40% of the world’s trash is burnt, and the majority of municipal solid waste contains about 12% different types of plastics [30].
Dioxins, furans, and polychlorinated biphenyls are among the harmful gases released into the atmosphere when plastics are burned. It damages human and animal health and vegetation [9]. Waste disposal in landfills indicates an unrecoverable loss of energy and precious raw resources. PVC produces carbon black, dioxins, and aromatics like chrysene and pyrene. Further, polypropylene (PP), polyethylene (PE), polystyrene (PS), etc., incomplete open combustion can release high amounts of carbon monoxide (CO) and noxious pollutants.
Additionally, plastic burning produces solid residual ash and soot, which are airborne particle emissions. In particular, volatile organic compounds (VOCs), semi-VOCs, particulate matter, polycyclic aromatic hydrocarbons (PAHs), and dioxins have been linked in several studies to soot and solid residual ash as being very likely to be harmful to human health and the environment [9].
Mechanical Recycling
Waste plastic can mechanically be converted into new goods or secondary raw materials without appreciably altering the chemical composition of the original plastic. All thermoplastics can be recycled mechanically with some quality loss, depending on the frequency at that the product/batch is recycled. Mixing in virgin plastic and adding additives could raise the new product’s quality. Most plastic processing firms prefer to employ virgin material at the expense of the environment because the cost of the collecting, sorting, shipping, and recycling activities tends to make the process inefficient. Downcycling and upcycling are two categories for the mechanical recycling of plastic trash.
Plastic Waste Downcycling
In this approach, the recycled material consists of lower grade and performs less effectively than the virgin material. That’s why, unlike other substances like metal and glass, plastics cannot continue to serve almost the same purpose after recycling. This is typically the case with plastics that lose specific features with time, such as mechanical integrity, optical clarity, and other inherent qualities that make them unsuitable for their intended use. A plastic water bottle, for instance, might be recycled into plastic furniture or artificial grass. In general, mechanical recycling of plastics by heating and molding activities results in downcycling over time because the material eventually becomes unsuitable for products with demanding engineering specifications.
Plastic Waste Upcycling
Waste plastic is converted into new products or materials that are thought to be of higher quality, such as those with environmental or artistic value, through upcycling, also known as creative reuse. For instance, recycled plastic bottles can be made into kid’s toys, garden sprinklers, flower pots, bird feeders, green parking canopies, and more. As mentioned, downcycling occurs when waste plastics are heated and remolded because they can no longer be used in the most demanding technical applications. Waste single and mixed plastics can be upcycled through thermochemical techniques. A thermo-chemical process called pyrolysis is currently receiving a lot of attention.
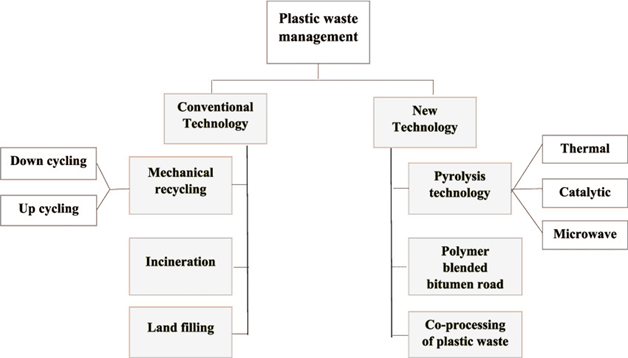
Figure 1: Conventional and new approaches to managing plastic waste
Pyrolysis
Over other traditional waste plastic management techniques, pyrolysis offers several benefits. For instance, most recycled waste plastics are downcycled, meaning they are utilized to make inferior quality products with fewer uses. This is because repeated recycling causes the plastic to lose qualities like clarity, strength, and flexibility.
Second, the pyrolysis approach fully eliminates the cost of washing, sorting, and mixing waste plastics prior to mechanical recycling.
Mechanical recycling entails melting and remolding discarded or used polymers into new products. This suggests that the only wastes appropriate for this approach are those from thermoplastic products. Since they cannot be remolded, the thermoset portion will thus continue to exist in the dumpsites. On the other hand, both thermoset and thermoplastic materials can be employed as feedstock in pyrolysis procedures [11]. The waste of many innovative materials, including composites, can also be treated using pyrolysis, particularly in the rapidly developing field of designed applications where polymer composites are increasingly replacing conventional materials.
Finally, the product and yield composition may be adjusted by changing the feedstock composition, process conditions (temperature, reaction gas, heating rate), and the presence (catalytic pyrolysis) or absence of a catalyst (thermal pyrolysis).
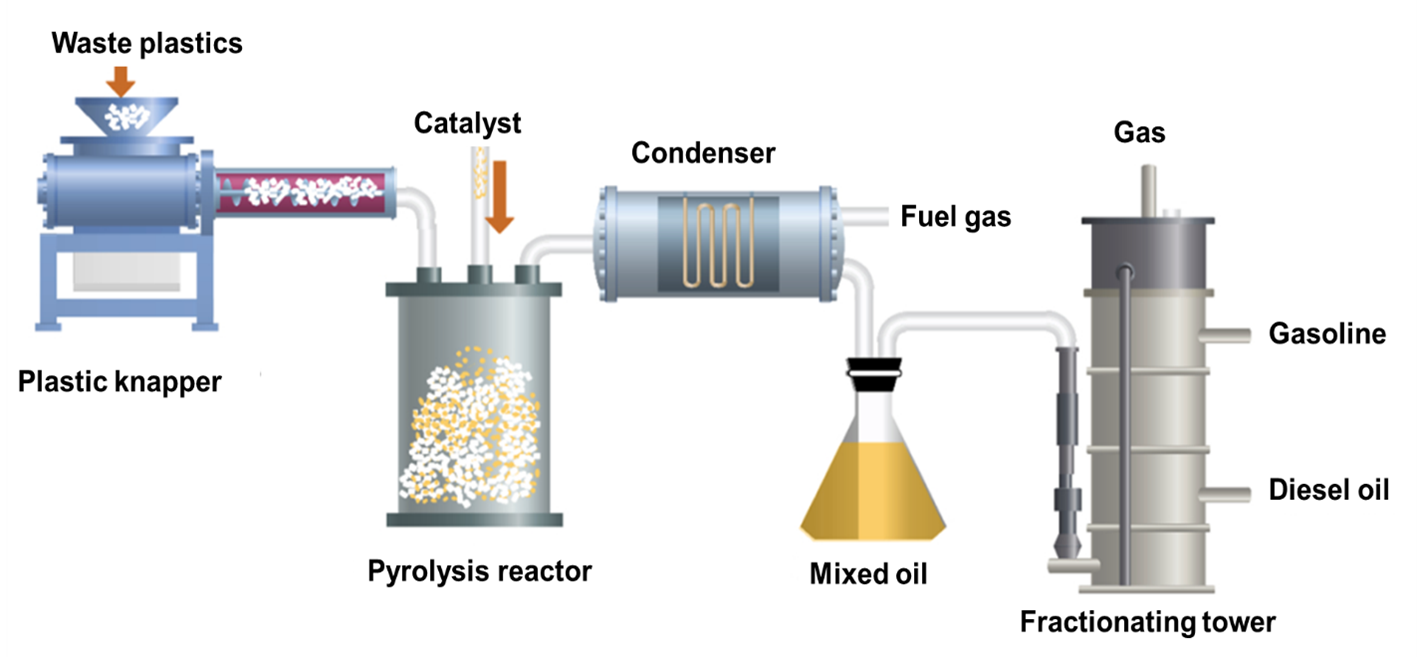
Figure 2: A simplified process of plastic waste pyrolysis
Pyrolysis Classification on Heating Rates
Slow Pyrolysis
The slow heating of the feedstock without oxygen is known as slow pyrolysis. The organic material partially evaporates rather than combust, leaving behind a substance called char, mostly (usually 80%) made of carbon. Slow pyrolysis, also known as carbonization, stresses the solid char as the primary result rather than the liquid one that occurs during fast pyrolysis. 10 °C/s is maintained as the heating rate.
Fast Pyrolysis
Fast pyrolysis produces a large volume of liquid pyrolysis fuel by quickly heating the feedstock to moderate temperature (400–600°C) with a short residence period (a few seconds). Fast pyrolysis creates an environment that maximizes liquid generation, and the reactor is thought to run isothermally. It is the approach used in plastics pyrolysis research and application most frequently. The heating rate of 100°C/s is maintained.
Ultra-fast/flash Pyrolysis
Flash pyrolysis refers to a thermal decomposition pyrolysis that occurs at a very high heating rate. Gases and bio-oil are the principal outputs. The range of heating speeds is 100–10,00°C/s, and residence periods are short.
Other Classification of Pyrolysis
Thermal Pyrolysis Technique
The process involves depolymerizing or breaking plastic materials by heating them to extremely high temperatures in an atmosphere with little or no oxygen. The temperature usually ranges from 350 to 900 °C. The results include char that has been carbonized, liquid, and gaseous. The volatile product’s condensable portion typically yields liquid fuel, while some noncondensable high calorific value gas often generates during the processes. Its liquid product combines paraffin, olefins, isoparaffins, naphthenes, and aromatic compounds. High temperatures of up to 900°C are necessary due to chain scission, bond breakage, intermolecular and intramolecular forces [12]. The kind of plastic significantly impacts the physical characteristics of the liquid fuel produced by this method [13].
Table 1: Liquid fuel properties of different single plastics after pyrolysis
| Plastic waste | Density (400C) g/cm3 | Viscosity (400C) cSt | Flashpoint (0C) | Calorific value (MJ/kg) | Appearance |
| LDPE | 0.77 – 0.8 | 1.6-1.8 | 50 | 39.1 | Brown |
| HDPE | 0.8 – 0.92 | 2.4 – 2.5 | 40-48 | 45.4 | Brown |
| PP | 0.77 – 0.8 | 2.7 | 31 – 36 | 40 | Yellow |
| PET | 0.087 – 0.9 | — | — | 28.2 | Brown |
| PS | 0.85 – 0.86 | 1.4 | 28 | 43 | Deep brown |
Microwave-assisted Pyrolysis Technique
Microwave dielectric heating is a component of microwave pyrolysis, commonly referred to as microwave-assisted pyrolysis. Depending on the dielectric qualities of the material, microwaves can interact with it in one of three ways: via reflecting off of conductors, transmitting through perfect insulators, or absorption and decaying inside the substance. Heat is produced by alternating electromagnetic fields that stir up the molecules in dielectric materials. The principle behind plastic microwave pyrolysis is the passage of thermal heat from an absorbent, which first absorbs microwave radiation, to the plastic. The physical characteristics of absorbent and volume ratio influence the uniformity of heating dispersion.
If we vary microwave strength, this results in entirely different product dispersion. Microwave-induced pyrolysis has been shown to have several benefits over thermal and catalytic pyrolysis and may be utilized to produce chemicals and fuels with added value. Plastics could be quickly, volumetrically, and arbitrarily heated using this method to recover energy. Plastics cannot absorb microwave radiation because they have an extremely low dielectric loss factor. As a result, the plastic needs to be combined with an absorbent to help in pyrolysis. High dielectric loss factors substances, such as carbon, iron mesh, and silicon carbide are suitable options for use as absorbents in plastic pyrolysis.
Table 2: Microwave pyrolysis of plastic waste using tires as microwave absorbent [14]
| Plastic | Microwave power (kW) | Time (min) | Absorbent-plastic ratio | Pyrolysis products (wt%) | ||
| Solid | Liquid | Gas | ||||
| HDPE | 3 | 75 | 1:2 | 0.4 | 83.9 | 15.7 |
| PP | 3 | 69 | 1:2 | 15.9 | 70.8 | 13.3 |
| PVC | 3 | 21 | 1:2 | 14.7 | 3.4 | 81.9 |
| PET | 1.8 – 3 | 40 | 2.5 | 38.2 | 35.3 | 26.5 |
| PS | 3 – 6 | 591 | 1:2 | 6.8 | 84.2 | 9 |
Catalytic Pyrolysis
Catalytic pyrolysis is a process whereby polymeric materials are broken down through the application of heat in the presence of a catalyst, all while excluding oxygen. In plastic pyrolysis, catalysts are used primarily to decrease the amount of energy needed, change the product’s composition by cracking, and speed up the process. Zeolites, silica-alumina, and fluid catalytic cracking are some of the most often employed catalysts for the pyrolysis of plastic waste. Zeolite-based catalysts are often more efficient than nonzeolitic catalysts because their superior acid strength produces a greater quantity of gaseous product. Studies have shown that the two-stage technique offers certain advantages in catalytic pyrolysis in comparison to one-stage breakdown.
- Effect of Catalyst Contact Mode
When plastics are pyrolyzed in a reactor, there are two main ways to apply catalyst: liquid (in-situ) and vapor phase contact (ex-situ). In in-situ, the polymer and catalyst are combined in a reactor and the temperature is raised to the required reaction level. Later, however, the polymer is first thermolyzed in order to generate the volatile portion. The hydrocarbon vapors are cracked to get the desired product distribution as the vapor travels past the catalyst, which is placed in the path of the flowing vapor. The research findings indicated that in-situ catalytic pyrolysis generated more aromatics than ex-situ catalytic pyrolysis, specifically for PE, PP, and polyethylene terephthalate (PET). Conversely, in the case of PS, ex-situ catalytic pyrolysis resulted in a higher yield of aromatics due to the greater production of styrene in the resultant products. Based on their findings, the researchers determined that the varying product compositions observed in the two modes of operation indicate that depolymerization of the polymers proceeds through different reaction mechanisms.
- Effect of Polymer/Catalyst Ratio
According to several studies, the polymer/catalyst ratio greatly impacts the composition and yield of plastic pyrolysis products. However, it may be inferred that there is no direct correlation between an increase in catalyst concentration and an increase in conversion or overall effectiveness. Increasing the catalyst quantity boosts conversion until a certain point, but increasing the catalyst %age further does not result in a noticeably higher conversion rate.
(c) Effect of Temperature
The pyrolysis process is strongly influenced by temperature. When catalytic pyrolysis occurs at elevated operating temperatures or rapid heating rates, it promotes the cleavage of chemical bonds and leads to the production of smaller molecules at a higher rate [15]. With increasing temperature, the degree of conversion also increases, leading to enhanced production of gaseous byproducts, which consequently reduces the yield of liquid products. As the temperature rises, the impact of various catalysts on the liquid yield and product range becomes less relevant. Under the same process conditions, catalytic pyrolysis of polymers occurs at a substantially lower temperature than thermal pyrolysis [16]. Under catalytic conditions, the cracking rate increased at lower temperatures.
- Effect of Gas Flow Rate
Nitrogen being an inert gas does not affect the pyrolysis process taking place inside the reactor. On the other hand, it speeds up the passage of volatiles into the condenser, making gases available for condensing into liquid fuel. The nitrogen flow rate is typically maintained at 10 mL/s or less for most plastic pyrolysis cases documented in the study [17]. Since it can turn different types of waste plastic into liquid fuel and effectively manage waste plastic while providing excellent economic benefits, pyrolysis technology is used more frequently today instead of landfill or other mechanical recycling approaches.
Table 3: Pyrolysis types, associated parameters and general products
| Pyrolysis methods | Residence time | Heating rate | Final temperature (ºC) | Major products |
| Fast | <2 s | ~100 ºC/s | 650 | Bio-oil |
| Flash | <1 s | >500 ºC/s | >650 | Gas, chemicals, bio-oil |
| Ultra-rapid | <0.5 s | Very high | 1000 | Chemicals, gas |
| Hydro-pyrolysis | <10 s | High | <500 | Bio-oil |
| Vacuum | 2-30 s | Medium | 400 | Bio-oil |
| Carbonization | Days | Very low | 400 | Charcoal |
| Conventional/slow | 5-30 min | ~10 ºC/s | 500 | Bio-oil, char, gas |
Advantages of Pyrolysis in dealing with Plastic Waste
High Heating Value
The liquid fuel produced by the pyrolysis of plastic waste is typically heavier than some commercial-grade fuel, which results in a higher heating value of 42 MJ/L or more [18]. This property benefits fuel used in cement plants, boilers, steel and glass mills.
Feed Selectivity
The pyrolysis technology can be used to process almost any type of waste plastic, clean or dirty, sorted or unsorted. There is no need to perform the shredding process for batch plastic waste pyrolysis. The waste pyrolysis reactor and its supporting infrastructure house the whole of the plastic-to-fuel conversion process, saving a lot of effort and time. There have also been reports of the co-pyrolysis of plastics with biomass [19], waste paper [20], plastics from healthcare waste, etc.
Pyrolysis of Mixed Plastic Waste
The ability to employ different classes and types of plastics as a single feedstock without sorting and disassembling is a significant benefit of pyrolysis technology for plastic waste management. Pyrolysis does not need the precise separation of various polymers, unlike recycling. Since most plastics do not mix effectively, they are unable to be treated together during recycling. For instance, just a small amount of PVC in the PET recycling stream may weaken and turn yellow the entire PET resin [21].
Self Sustainable Process
Plastics can be pyrolyzed to produce a hydrocarbon-rich gas with a thermal value of 25–45 MJ/kg [22], which is perfect for recovery of energy. As a result, in commercial-scale pyrolysis, the energy generated is fed back into the process to supply the needed energy, making the entire process self-sustaining.
Infrastructure Required
The pyrolysis technology allows for the installation of small, possibly movable units at waste concentration locations with a large supply of feedstock because it does not require significant infrastructure. Scalable pyrolysis options, for instance, can be found in populated areas in Finland with the proper environmental permissions [23]. This strategy not only avoids the expense of moving waste to centralized recycling facilities but also lowers the value chain’s carbon impact.
Product Selection
The pyrolysis process allows for product tuning by changing operating conditions, reactor type, and the use of a catalyst and type. Regarding the overall process’s finances, this is very beneficial. Although the primary objective of the process is typical to maximize the output of pyrolysis liquid, the process can be modified to maximize the production of wax, monomers, aromatic compounds, or selective compounds with the use of an appropriate catalyst.
Industrial Integration
The oil and paraffin derived from the pyrolysis of waste plastic are rich in hydrocarbons, making them ideal refinery feedstock. Numerous studies have been conducted to model the incorporation of polymers pyrolysis into traditional refineries. Additionally, the combination has been tested, for example, at the ReOil pilot plant at the OVI oil refinery in Austria [24], where plastic waste is pyrolyzed and the resulting liquid is supplied to the refinery unit. Although the size is currently small in comparison to unrefined oil, the proof of concept has been established. This demonstrates how a circular economy for polymers can be closed using thermochemical recycling.
Pyrolysis Technical Challenges
Feedstock Availability
Despite advanced technologies, one of the primary reasons many plastic recycling projects have failed is the lack of continuous availability of plastic waste. A constant supply of feedstock with uniform quality is necessary for the pyrolysis of plastic waste to be financially viable. Large feedstock quantities are also necessary for economics. In the past, EU countries sent a sizable volume of plastic waste to China. China, however, has ceased importing waste plastic. As a result, substantial amounts of waste plastic need to be handled locally in EU member states. To address this transition, the EU member states have introduced several initiatives, rules, and programs aimed at boosting plastic recycling rates by introducing novel recycling techniques. While having a waste supplier in the recycling value chain is crucial. As in the EU countries plastic waste has to be treated locally, significant changes to waste recycling value chains, it is likely that the lack of available feedstock will not pose a significant issue.
Selection of Feedstock
Plastics gathered from various waste sources are quite diverse. A large portion of plastic products are made of polyolefin; however, PVC and PET are also present in some streams. Polyolefins (PE and PP) make an ideal feed for pyrolysis because of their chemistry and synergistic effects, while PVC and PET create issues in the feed. When PVC degrades thermally, chlorine-containing chemicals are released, which lead to equipment corrosion and halogenation of the oil. However, the packaging business uses a lot of PET and recycling PET is a well-known practice. This intends the separation of PET plastics from other types of plastic waste. Sometimes this separation comes with a substantial cost factor.
Pretreatment
Plastic products come in a variety of forms and sizes; thus, they must be evenly sized before being fed into the pyrolysis process. The cost of this extra step is added to the overall process cost.
Material Feeding Constraints
Feeding plastic trash during the pyrolysis process is difficult because of its irregular forms and sizes. A variety of feeding devices have been tested to meet this difficulty. Screw feeders are used in some facilities, and hydraulic feeding systems are prevalent. Plastic films are challenging to feed via screw feeders because they frequently wrap themselves around the screw. They also generate a melt on the hot screw, making it challenging for the system to carry on feeding. So, a sophisticated approach in terms of screw design and the nature and size of the feeding stream is required.
Feedstock Toxicity
In order to carry out successful pyrolysis of plastic waste, it is crucial first to perform a thorough characterization of the feedstock. This is due to the highly heterogeneous nature of plastic waste, which can be derived from a wide range of sources and may contain different toxic ingredients such as halogens, harmful additives, biohazardous materials and other hazardous components. Releasing these toxic materials from recycling poses a significant threat to the environment and human health. Therefore, proper characterization of the feedstock is necessary to ensure the production of high-quality end products while maintaining operational safety.
Reactor Selection
The reactor selection greatly influences the pyrolysis product spectrum. Reactors for pyrolysis can be broadly divided into rapid and slow classifications. There are advantages and disadvantages to each type of pyrolysis reactor, but the decision must be made depending on the required product and the makeup of the accessible feedstock. Heat and mass transmission are the two key aspects that must be considered while selecting the right reactor type.
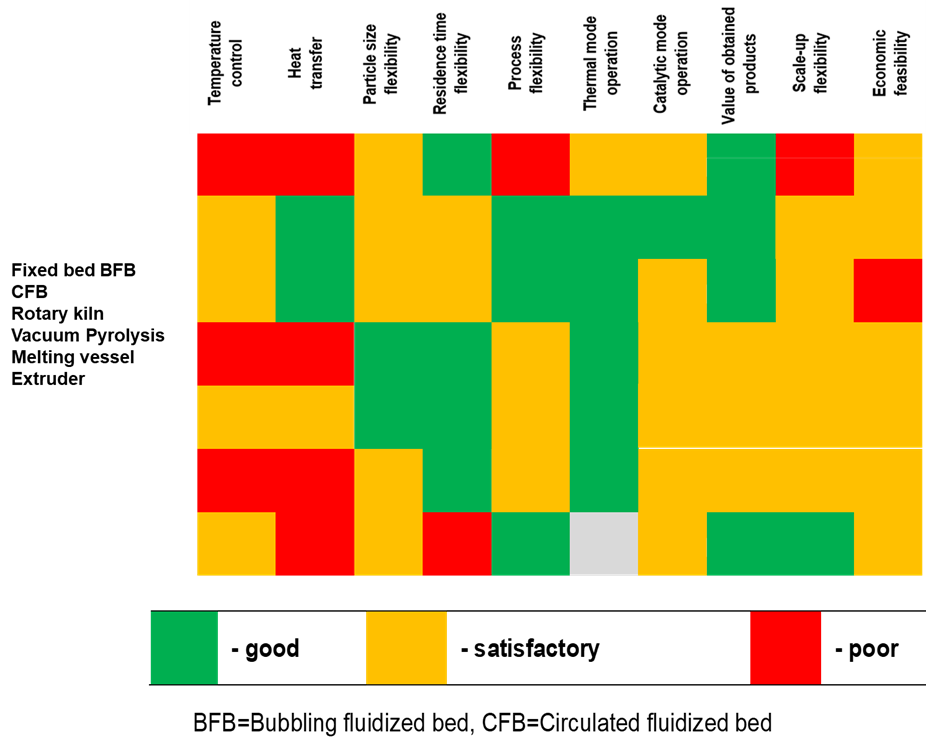
Figure 3: Plastic pyrolysis reactor comparison (adopted from [25])
Wax Formation
Wax is produced during the pyrolysis of plastics, particularly polyolefins, and is one of the main byproducts. The recovery systems should be built to manage waxes in order to retrieve them easily. Recovery is challenging because ineffective condensing systems scale wax on the inner surfaces. When wax is the interested end product, the recovery system must be specifically developed to handle waxes from plastic pyrolysis. It can be very challenging to recover material during the process when using typical condensing systems because wax frequently condenses on the wall of the condenser.
Analytical Standards
Since pyrolysis liquid made from plastic waste is not a standardized product, very few standard testing procedures are available. The intricacy and heterogeneity of waste also contribute to the lack of testing procedures. It is challenging to develop standards because the pyrolysis liquid’s composition varies greatly depending on the feedstock’s makeup. Nevertheless, despite these difficulties, efforts are being made to design the procedures for examining the product in order to achieve a standard product status.
Long-term Stability and Storage
The stability and aging characteristics of pyrolysis liquids are important factors in determining the liquid’s quality. Pyrolysis liquid tends to repolymerize and is thermodynamically unstable. In order to preserve the quality of the liquid over an extended period of time, post-treatment is required. These post-treatment options include blending, dewaxing, and others [51]. This step comes with additional complications and associated costs.
Is Plastic Pyrolysis Sustainable?
So, a question will come to mind is plastic pyrolysis a sustainable and economical approach to plastic disposal? Plastic pyrolysis is a useful method for getting rid of waste plastic, but it also has drawbacks. For instance, the pyrolysis of plastic releases toxic gases like PVC, which is itself safe for the atmosphere. It emits a lot of carbon, gases like CO2 and water vapor are released by it.
While reading this might lead you to believe that it is not sustainable; pyrolysis releases 1/30 of the greenhouse gases that landfills do. In order to produce fuel and virgin plastic resins, it also significantly reduces the need to mine crude oil and gas from the earth. Dioxins are also not released during pyrolysis, unlike when plastic waste is burned in landfills.
Pyrolysis is a green method of recycling plastic and capturing fuel energy. Pyrolysis is not only less polluting but also cost-effective. Because the raw material, which is plastic waste, is readily accessible and getting bigger every year.
Conclusion and Future Perspective
We are currently well-informed about the environmental issues brought on by waste plastics, which range from issues with landfilling to pollution—including coastal pollution—and even groundwater contamination. Due to man’s growing reliance on plastics for everyday use, which is made possible by their strength, flexibility, resilience to moisture, lightweight nature, and comparatively low cost, these issues have only continued to grow immensely.
Pyrolysis uses discarded plastics as feedstock to create energy and other useful products, promoting the circular economy and changing our surroundings to be beautiful and helpful. Another school of thought, however, challenges this technology as being unsustainable and, therefore, ineffective in addressing the issues associated with discarded plastics. However, this study refuted this by reviewing numerous works that made this claim, pointing out the significant environmental benefits of pyrolysis over other waste plastic recycling techniques in line with their distinctive characteristics of not producing dioxins and having lower CO and dioxide emissions.
The future perspective of the pyrolysis process is promising due to its potential to address several key challenges facing society today, including climate change, waste management, and energy security. Here are some of the technicalities that could play a role in the future of pyrolysis:
- Advancements in pyrolysis technology: There is ongoing research to improve the efficiency of pyrolysis reactors, increase the yield of desired products, and reduce the cost of production. This includes the development of new reactor designs, such as fluidized beds and microwave-assisted pyrolysis, and the use of catalysts to increase the yield of bio-oil.
- Integration with other technologies: Pyrolysis can be combined with other technologies, such as gasification and combustion, to produce a wider range of products and increase overall efficiency. For example, syngas produced from pyrolysis can be used as a feedstock for gas turbines to generate electricity.
- Carbon capture and storage: Pyrolysis can be used in combination with carbon capture and storage (CCS) technologies to reduce greenhouse gas emissions. Pyrolysis can become a carbon-negative technology by capturing and storing the carbon dioxide generated during the process.
- Policy and market incentives: Government policies and market incentives, such as carbon pricing and renewable energy standards, can drive the adoption of pyrolysis technology. As these incentives become more common, pyrolysis could become an increasingly attractive option for biomass conversion.
Overall, the future perspective of pyrolysis is positive, as the technology has the potential to contribute to a more sustainable and circular economy. However, more research and development are needed to fully realize the potential of pyrolysis as a key technology in the transition to a low-carbon economy.
Endnotes
[1] Chalmin, P. (2019). The history of plastics: from the Capitol to the Tarpeian Rock. Field actions science reports. The Journal of Field Actions(Special Issue 19), 6-11.
[2] Gaiya, J. D., Eze, W. U., Oyegoke, T., Alewo, A., Madufor, I., and Bello, T. (2021). Assessment of the dielectric properties of polyester/metakaolin composite. Eur J Mater Sci Eng, 6, 19-29.
[3] Babayemi, J. O., Nnorom, I. C., Osibanjo, O., and Weber, R. (2019). Ensuring sustainability in plastics use in Africa: consumption, waste generation, and projections. Environmental Sciences Europe, 31(1), 1-20.
[4] Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Science advances, 3(7), e1700782.
[5] Ratnasari, D. K., Nahil, M. A., and Williams, P. T. (2017). Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. Journal of analytical and applied pyrolysis, 124, 631-637.
[6] Srivastava, V., Ismail, S. A., Singh, P., and Singh, R. P. (2015). Urban solid waste management in the developing world with emphasis on India: challenges and opportunities. Reviews in Environmental Science and Bio/Technology, 14, 317-337.
[7] Eze, W. U., Madufor, I. C., Onyeagoro, G. N., Obasi, H. C., and Ugbaja, M. I. (2021). Study on the effect of Kankara zeolite-Y-based catalyst on the chemical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polymer Bulletin, 78(1), 377-398.
[8] Schug, T. T., Janesick, A., Blumberg, B., and Heindel, J. J. (2011). Endocrine disrupting chemicals and disease susceptibility. The Journal of steroid biochemistry and molecular biology, 127(3-5), 204-215.
[9] Verma, R., Vinoda, K., Papireddy, M., and Gowda, A. (2016). Toxic pollutants from plastic waste-a review. Procedia Environmental Sciences, 35, 701-708.
[10] Conlon, K. (2021). A social systems approach to sustainable waste management: leverage points for plastic reduction in Colombo, Sri Lanka. International Journal of Sustainable Development & World Ecology, 28(6), 562-580.
[11] Eze, W. U., Madufor, I. C., Onyeagoro, G. N., and Obasi, H. C. (2020). The effect of Kankara zeolite-Y-based catalyst on some physical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polymer Bulletin, 77, 1399-1415.
[12] López, A., De Marco, I., Caballero, B., Laresgoiti, M., and Adrados, A. (2011). Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chemical Engineering Journal, 173(1), 62-71.
[13] Santaweesuk, C., and Janyalertadun, A. (2017). The production of fuel oil by conventional slow pyrolysis using plastic waste from a municipal landfill. International Journal of Environmental Science and Development, 8(3), 168.
[14] Undri, A., Rosi, L., Frediani, M., and Frediani, P. (2011). Microwave heating. Microwave pyrolysis of polymeric materials. London: IntechOpen.
[15] Miskolczi, N., Angyal, A., Bartha, L., and Valkai, I. (2009). Fuels by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Processing Technology, 90(7-8), 1032-1040.
[16] Chen, D., Yin, L., Wang, H., and He, P. (2014). Pyrolysis technologies for municipal solid waste: a review. Waste management, 34(12), 2466-2486.
[17] Cleetus, C., Thomas, S., and Varghese, S. (2013). Synthesis of petroleum-based fuel from waste plastics and performance analysis in a CI engine. Journal of Energy, 2013.
[18] Panda, A. K., Alotaibi, A., Kozhevnikov, I. V., and Shiju, N. R. (2020). Pyrolysis of plastics to liquid fuel using sulphated zirconium hydroxide catalyst. Waste and Biomass Valorization, 11, 6337-6345.
[19] Supramono, D., Nabil, M., and Nasikin, M. (2018). Effect of feed composition of co-pyrolysis of corncobs–polypropylene plastic on mass interaction between biomass particles and plastics. IOP Conference Series: Earth and Environmental Science,
[20] Sophonrat, N. (2019). Pyrolysis of mixed plastics and paper to produce fuels and other chemicals KTH Royal Institute of Technology].
[21] Hopewell, J., Dvorak, R., and Kosior, E. (2009). Plastics recycling: challenges and opportunities. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1526), 2115-2126.
[22] Oasmaa, A., Qureshi, M. S., Pihkola, H., Ruohomäki, I., Raitila, J., Lindfors, C., Mannila, J., zu Castell-Rudenhausen, M., Deviatkin, I., and Korpijärvi, K. (2019). Fast pyrolysis of industrial waste residues to liquid intermediates-experimental and conceptual study.
[23] Louis, J.-N., Caló, A., Pongrácz, E., and Hänninen, N. Micro Energy.
[24] ReOil: Getting crude oil back out of plastic. https://www.omv.com/en/blog/reoil-getting-crude-oil-back-out-of-plastic
[25] Arena, U., and Mastellone, M. L. (2006). Fluidized bed pyrolysis of plastic wastes. Feedstock recycling and pyrolysis of waste plastics: converting waste plastics into diesel and other fuels, 435-474.

 To all knowledge
To all knowledge